A Comparative Study on Antiradical and Antimicrobial Properties of Red Grapes Extracts Obtained from Different Vitis vinifera Varieties ()
1. Introduction
Phenolic compounds make up one of the major families of secondary metabolites widely distributed in the plant kingdom, and they are found in foods of vegetable origin, constituting an integral part of our daily diet [1]. Grapes contain large amounts of phenolic compounds in skins, pulp and seeds, which are partially transferred to wine during red wine-making [2,3]. Currently, grape compounds have attracted increased attention especially in the field of nutrition, health and medicine.
Structurally, phenolic compounds comprise an aromatic ring, bearing one or more hydroxyl substituents, and range from simple phenolic molecules such as phenolic acid to highly polymerized compounds such as tannins [4]. Phenolic compounds are divided into several classes according to the number of phenol rings that they contain and to the structural elements that bind these rings to one another [5]. Phenolic grape and wine compounds can be divided into two groups: non-flavonoid (Hydroxybenzoic and Hydroxycinnamic acids, Stilbenes) and flavonoid compounds (Anthocyanins, Flavan-3-ols and Flavonols) [6].
Phenolic compounds are considered to be the most important components of red wine, due to their direct relationship with its color, astringency, bitterness, and susceptibility to oxidation reactions [7]. Furthermore, phenolic compounds are known by their potent antioxidant, antimutagenic, antibacterial, antiviral, antifungal and antiulcer activities [8].
Indeed, due to the mobility of the phenolic hydrogen, phenolic compounds are able to scavenge free radicals generated continuously by endogenous factors such as normal physiological metabolism and also by exogenous factors including smoking, pollution, infections, sun exposure and others [9]. The loss of hydrogen causes the formation of a highly stabilized mesomeric radical.
Several studies have highlighted that phenolic compounds by virtue of their antioxidant capacity participate in the prevention of various chronic diseases in which an oxidative stress is potentially involved [10].
Nowadays bacteria, yeasts and free radicals cause real health problems because of their involvement in many diseases mainly those in which an oxidative stress is involved such as cancer and cardiovascular disease and in food borne diseases [11]. Currently there is a growing scientific interest to use natural antibacterial compounds, as biopreservatives face to conventional synthetic additives, due to consumer preferences towards more natural and healthier products [12]. Thus the role of natural phenolic compounds extracted from plant reaches its paroxyme and the addition of these natural compounds to food products has therefore become popular as a means of increasing shelf life and to reduce wastage and nutriational losses by inhibiting microbial growth and delaying oxidation [13].
The aim of the present work was to assess the antiradical and antimicrobial activities of phenolic compounds extracted from grapevine varieties of Château KSARA-Bekaa-Lebanon. Several previous studies have been conducted to evaluate antioxidant activity as well as antimicrobial activity of phenolic extract from wines or grapes seeds, skins and pulps [14-16]. As a first study in Lebanon two of the most important biological activities of phenolic compounds, antiradical and antimicrobial, have been tested on phenolic extracts prepared from the whole grape berries. The phenolic content of these grape extracts was determined by the Folin-Ciocalteau method. An HPLC-DAD method was conducted for the determination and quantification of the main phenolic compounds belonging to flavonoids and non-flavonoids molecules in order to determine the influence of these molecules towards their bioactive properties. The evaluation of antiradical activity was based on the capacity of a sample to scavenge the DPPH radical. In this work, we describe a new and innovative quantitative method for the evaluation of the antimicrobial activity of red grapes phenolic compounds against various pathogenic strains, such as Gram-positive and Gram-negative bacteria and yeasts.
2. Materials and Methods
2.1. Plant Material
The grapes examined in our study were harvested in the vintage 2009, at optimum maturity from vineyards in the province of Bekaa-Château KSARA S.A.L, Lebanon. At their optimum phenolic maturity, these grapes have both, a high content of phenolic compounds and a good extractability [17].
All samples analyzed were V. vinifera species from different cultivars. The varieties chosen were: Merlot, Syrah, Cabernet franc and Cabernet Sauvignon, the most important red grape varieties that can be encountered in the Lebanese vineyard. We should notice that this study is applied for the first time in the Lebanese vineyard.
2.2. Chemicals and Reagents
Solvents used for high-performance liquid chromatographic analysis were: Methanol (Merck, Darmstadt, Germany) and Formic acid (Scharlau, Barcelona, Spain) of HPLC ultra gradient grade. HPLC grade water (Merck, Darmstadt, Germany) was also used. Folin-Ciocalteu’s phenol reagent; Sodium Carbonate; 2,6-di-tert-butyl-4- methylphenol (BHT); 2,2-diphenyl-bpicrylhydrazyl(DPPH) radical and Tris-HCl buffer were obtained from Sigma (Aldrich).
Phenolic Standards: Gallic acid, Protocatechin, Hydroxybenzoic acid, Catechin, Epigallocatechin, Caffeic acid, Chlorogenic acid, Epicatechin, p-Coumaric acid, Gallocatechin gallate, Ferulic acid, Resveratrol, Cinnamic acid, Rutin, Myricetin, Quercetin and Kaempferol were purchased from Sigma (Aldrich) Laboratories.
2.3. Microbial Strains and Growth Conditions
Antimicrobial activity was screened against two Gram negative bacteria: Escherichia coli ATCC 10536 (E. coli) and Salmonella arizonae ATCC 13314 (S. arizonae), one Gram positive bacteria: Listeria monocytogenes ATCC 19111 (L. monocytogenes), and one yeast strain: Candida albicans ATCC 10231 (C. albicans).
Chloramphenicol (Sigma, Aldrich) and Amphotericin B (Sigma, Aldrich) were used respectively as the reference antimicrobial and antifungal standards.
All strains were cryo-preserved at −80˚C. E. coli was cultured in Luria Broth, S. arizonae in Trypticase Soy Broth (BioMérieux, Marcy L’Etoile, France), L. monocytogenes in Trypticase Soy Broth-Yeast Extract (BioMérieux, Marcy L’Etoile, France), while the yeast strain C. albicans was maintained in Yeast Glucose Chloramphenicol Broth.
For experimental use, the microorganisms were sub cultured in agar media, incubated for 24 h at 37˚C for bacteria and 30˚C for yeast and used as the source of inoculums for each experiment. After incubation, each microorganism was suspended in 5 mL of appropriate broth and incubated for one hour at adequate temperature with agitation. Cultures growth was followed by the turbidity measurement at 630 nm, until it achieved the turbidity of 0.5 McFarland standard, which is equal to an inoculum containing approximately 108 cfu/mL [18].
2.4. Sample Preparation and Extraction
In our study, 239 samples of red grapes extracts from different varieties were analyzed and quantified. According to their phenolic maturity, four samples presenting the greatest phenolic maturity were selected. The phenolic constituents from the whole grape berries were extracted using conventional solvent extraction procedure. The extraction protocol was developed and optimized in our laboratory [19]. Briefly, to extract phenolic compounds, ten grams of homogenized grape berries (in high speed grinder) were mixed with 15 mL of acetone solvent (acetone/water 85/15, v/v) at room temperature. Contact time was 3 to 4 days. When the extraction is completed, the mixture is centrifuged at 2500 rpm for 15 minutes, at 20˚C. When completed, the supernatant was recovered and transferred into new tubes and incubated for two days at 40˚C to ensure the complete evaporation of the solvent. After the extraction, samples were filtered through 0.2 mm syringe filters. The resulting extract was stored at −20˚C protected from light.
2.5. Determination of Total Phenolic Compounds Content Using Folin-Ciocalteau Method
The total phenolic content was determined according to Folin Ciocalteu (FC) method [20]. An aliquot of 10 µl of the sample solution was mixed with 100 µl of commercial Folin-Ciocalteau reagent and 1580 µl of water. After a brief incubation at room temperature (5 min), 300 µl of saturated sodium carbonate was added. The color generated was read after 2 h at room temperature at 760 nm using a UV-Vis spectrophotometer (UV-9200, BioTECH Engineering Management, UK). A calibration plot of absorbance versus phenol concentration was made using Gallic acid as standard. The total phenolic compounds content in samples was evaluated from the generated absorbance value.
2.6. Determination of Anthocyanin Concentration
The concentration of free anthocyanins in red grapes extracts selected was analyzed by bleaching with bisulphite [21]. The bisulfite bleaching procedure requires the preparation of two samples, each containing 1 ml of grape extract, 1 ml of ethanol (EtOH), 0.1% Chlorydric acid (HCl) and 20 ml of HCl at 2% (pH 0.8). For the two samples, 4 ml of water (H2O) are added to 10 ml of the first sample, 4 ml of sodium bisulfite solution, are added to 10 ml of the second sample and the mixture is diluted by half. The difference in optical density (ΔOD) at 520 nm is measured on a 1 cm optical path. By comparison with a standardized anthocyanin solution, the concentration is given by the following equation:
C (mg/l) = ΔOD × 875
875 is the slope of the calibration curve obtained from Malvidin-3-glucoside.
2.7. HPLC Analysis
Polyphenol analyses of the extracts prepared from the whole red grapes were performed by high-performance liquid chromatography (HPLC). Prior to analytical chromatography, samples and standards were purified by filtration through 0.2 mm syringe filters to remove interferences of sugars and organic acids from the crude sample. An equipment consisting of a liquid chromatographyKNAUER apparatus coupled to a diode array detector was employed. Analyses were performed on a Spherisorb ODS-2 (5 mm, 250 × 4.6 mm), at a flow rate of 1 mL·min−1, using a 20 µL injection volume, detection at 280 nm and 320 nm, and the elution programme given in Table 1. Eluent A was 2% aqueous Formic acid and eluent B: 69% Methanol (MeOH), 29% HPLC water and 2% Formic acid. Identification was based on comparing retention times of the peaks detected with those of originnal compounds, and on UV-Vis on-line spectral data. Quantification was accomplished using the phenolic standards solutions. Results were expressed as mg/ml grape extract volume [22].
2.8. Biological Activity of Grape Extracts
The antiradical and antimicrobial capacity of phenolic compounds, in a general was well known [23,24]. As previously described, individual phenolic compounds present in grape extracts were identified and quantified, but we choose to submit the entire extracts to the biological activity studies. In fact, total food extracts may be

Table 1. Linear gradient used for the separation of phenolic compounds present in grapes.
more beneficial than isolated constituents, since a bioactive individual component can change its properties in the presence of other compounds present in the extract [25] corresponding to a synergistic effect.
2.8.1 Free Radical Scavenging Activity
The scavenging activity of DPPH free radical by grape must was determined according to the method reported by [26,27]. The free radical scavenging activity of extracts were examined by comparing to those of known antioxidants such as butylhydroxytoluene (BHT) (a synthetic antioxidant) and resveratrol (a natural antioxidant) by 1,1-diphenyl-2-picrylhydrazyl (DPPH). Each extract or positive control (BHT or Resveratrol) was diluted at a series of extract concentrations of 1, 5, 10 and 50 µg/mL. In each reaction, an aliquot of 50 µL of the diluted extract was added to 3.9 mL of DPPH solution in Methanol (0.1 mM) and 450 mL of Tris-HCl buffer. Absorbance at 517 nm was measured after 30 min of incubation at room temperature using pure Methanol as a blank. All samples were analyzed in duplicate.
The percentage of inhibition of the DPPH was determined as follows:
% inhibition = [(absorbance of control – absorbance of test sample)/absorbance of control] × 100
The free radical scavenging activity of Lebanese grape extracts was evaluated by the decrease in the peak area of the DPPH radical which exhibits a deep purple color with maximum absorption at 517 nm. Antioxidant molecules can quench DPPH free radicals, resulting in decoloration of DPPH because of their conversion into a colorless product.
2.8.2. Antimicrobial Activity
To determine the antimicrobial effect of the grape extracts, a new quantitative method was adapted. Aliquots of 200 µL bacterial or fungal pure cultures previously prepared (L. monocytogenes or S. arizonae or E. coli or C. albicans) were mixed with 200 µL of each sample or antimicrobial agent (Chloramphenicol), antifungal agent (Amphotericin B) and phenolic standards (Resveratrol, BHT, Gallic acid) used as positive controls at a concentration of 5 mg/mL and grown at 37˚C (for bacterial strains) and 30˚C (for fungal strain) for 24 hours with agitation. A negative control was also applied under the same experimental conditions, by replacing the grape extracts with the adequate broth for each microbial strain.
After appropriate incubation, serial dilutions from stock solutions were prepared in adapted broth for each strain. 500 µl from the 10−7 dilution was suspended into 20 mL agar medium and homogenized, then transferred into Petri dishes.
All plates were incubated for 24 h at 37˚C for bacterial strains and 30˚C for the fungal strain. After incubation the numbers of bacteria or yeast colonies that grow on each plate were counted.
The inhibitory effect was calculated using the following formula:
% Inhibition = (1 − T/C) × 100,
Where T = cfu/ml of test sample and C = cfu/ml of negative control [28].
2.9. Statistical Analysis
Each modality was conducted in duplicates and analysis repeated twice. Means and standard deviations of data were calculated. Two way analysis of variance (ANOVA) followed by Fisher’s LSD (Least Significant Difference) were performed to compare the means of the different investigated responses and to determine statistical significance. For each analysis, significance level of 5% was assumed. All statistical analyses were performed using Statgraphics 5.1 (Statpoint Technologies Inc., USA).
3. Results and Discussion
3.1. The Content of Polyphenols and Anthocyanins in Grape Extracts
The phenolic composition is an important quality parameter of red grape, which affects the quality of the resulting wines. Its knowledge is essential to classify red grapes varieties. In our study, 239 samples of red grapes extracts from different varieties were analyzed and quantified. Among these extracts, four were chosen for their high phenolic content and antiradical activities.
In order to classify the Lebanese grape varieties, a prior analysis of the phenolic and anthocyanin content of these four grapes extracts was done. Table 2 presents the total concentration of phenolic compounds (mg GAE/L) and anthocyanins concentrations (mg/L) of the Lebanese red grapes extracts (Merlot, Syrah, Cabernet Sauvignon and Cabernet Franc) used in this study. Based on statistical analysis by Fisher’s LSD test. Table 2 showed significant differences between phenolic content of the different grapes cultivars. Results showed that the total phenolic content of the different varieties was quite variable. This result was also proved [29], within a study on the polyphenolic content of different grape varieties. The variability found in total phenolic content between different cultivars confirmed the hypothesis that genetic and environmental factors [30-32], are key influencers of a cultivar’s phenolic content.
Table 2 showed that the anthocyanin concentration is also dependant from the variety. However, statistical analysis showed that only Syrah presents significant differences comparing to the other varieties. Whereas, nonsignificant differences were noticed between anthocyanin concentration contents of the other cultivars (Merlot, Cabernet Sauvignon and Cabernet Franc). This result confirmed the hypothesis that apart from the genetic background, several agroecological factors, such as maturation [33], ripening stage [34], cultivar [31], climate [35], stress levels [32], soil conditions vine water status and cultural practices) are able to impact the level of polyphenols and anthocyanin contents of red grape extracts. Therefore, these factors can be used to modify the phenolic composition of red grapes, which explains the differences between the phenolic and anthocyanin concentration for different red grape varieties.
Every family of polyphenols is directly responsible for the special characteristics of specific grapes varieties. In order to explain the physiological activities of phenolic compounds present in our different red grape extracts, an identification and quantification of these compounds were done before testing their antiradical and antimicro0 bial activities. Therefore, different phenolic compounds flavonoids and non-flavonoids were chosen as standards because of their biological and pharmacological interest and their contents were determined by reverse-phase HPLC (Tables 3 and 4). The concentration of the components was calculated from each chromatogram peak area.

Table 2. The weight (g) of grape berries, the phenolic (mg GAE/L) and the anthocyanin (mg/L) contents in the red grape varieties. The given values are the means of two repetitions.

Table 3. Non-flavonoid contents of the red grape varieties. (-) corresponds to a none-determined quantity.

Table 4. Flavonoid contents of the red grape varieties. (-) corresponds to a none determined quantity.
HPLC analysis of phenolic compounds in red grape extracts showed that most of the non-flavonoid and flavonoid compounds chosen have been identified in the different red grapes varieties of vintage 2009. Table 3 showed that the amount of non-flavonoid content and especially resveratrol is dependent on the red grapes varieties and that the Cabernet Sauvignon variety was richer in resveratrol than other cultivars, which was confirmed by Sun et al., 2006 [36]. The latter confirmed that stilbenes (Resveratrol) content is largely dependent of grape varieties. The phenolic pattern of grape extracts contains Cholorogenic acid, Caffeic acid, Gallic acid, Ferulic acid, Hydroxybenzoic acid, Cinnamic acid, pCoumaric acid and Protocatechuic acid. The concentration of each of these compounds varies between different cultivars.
Moreover Table 4 showed that the flavonoid content of grape extracts was variety dependent as well. The phenolic pattern of grape extracts contains Quercetin, Kaempferol, Myricetin, Rutin, Catechin, Epicatechin, Epigallocatechin, and Gallocatechin gallate.
Also Table 4 showed that Merlot and Cabernet Sauvignon varieties were the only cultivars that contain Catechin. This result was confirmed by other authors who showed that Cabernet Sauvignon and Merlot were the cultivar in which the Catechin is the main compound [37, 38].
To determine the total phenolic content of the grapes varieties, the total of flavonoid, non-flavonoid and phenolic acid contents of the four red grapes varieties were calculated (Table 5). Table 5 showed that flavonoid, non-flavonoid and phenolic acid contents were significantly different between the four grape varieties. Of these compounds, non-flavonoid-compounds especially phenolic acids are in higher concentration than the flavonoid compound at the exception of the variety Merlot KAM 33.
Table 5 showed that the greatest range of non-flavonoid was found in the grape extract of Cabernet Sauvignon TACS22 and the lowest in the grape extract of Merlot KAM33, with high flavonoid content for both cultivars. Therefore to present a global overview on the phenolic profiles of TACS22 and KAM33, two representative chromatograms of these two samples are illustrated in Figure 1.
Figure 1(a) represents the chromatogram for the Merlot KAM33 and Figure 1(b) for the Cabernet Sauvignon TACS22. The results showed that non-flavonoid compound especially Resveratrol were present in greater amounts in Cabernet Sauvignon TACS2 than in Merlot KAM33.
3.2. Radical Scavenging Effect Assay
The free radical scavenging activity of red grape extracts was assessed by DPPH assay. From a methodological point of view, the DPPH (2,2,-diphenyl-1-picryhydrazyl) method [39], is recommended as easy and accurate methods for measuring the antiradical activity of fruit and vegetable juice or extracts.
The scavenging effects of ethanolic extracts from red grapes from different varieties were examined and compared. Several concentrations of red grapes extracts ranging from 1 - 50 µg/mL were tested for their scavenging activity in vitro model. As shown in Figure 2, the amount of DPPH decreased in the presence of grape extracts. Therefore, radical scavenging activity increased with increasing percentage of the free radical inhibition. Figure 2 showed also that free radicals were scavenged by the test compound in a concentration dependent manner in the model. Figure 2 showed that all the test samples have a significant inhibitory activity against the DPPH radical, but the higher inhibition of DPPH radical by extracts samples was observed at a range of 50 µg/mL. This result is in agreement with the study of the scavenging activity of the seed methanolic extracts of three Vitis vinifera realized by Saïdani Tounsi M. et al. 2009 [40], who showed that all seed extracts showed remarkable DPPH radical scavenging activity at a concentration of 50 µg/mL.
Figure 2 showed also that the scavenging activity was different between the grape varieties, which was due to the difference in their phenolic compounds contents.
To find the contribution of different phenolic content

Table 5. Flavonoid, non-flavonoid and phenolic acid contents of the red grape varieties.
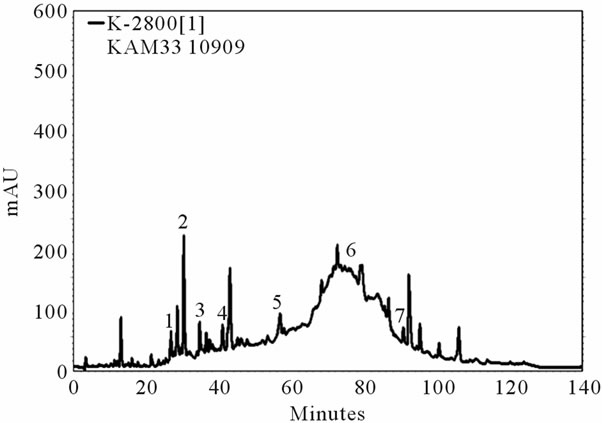 (a)
(a) (b)
(b)
Figure 1. HPLC chromatogram of red grape extracts of two varieties (a) Merlot KAM 33 and (b) Cabernet Sauvignon TACS22. Peak identification: 1. Hydroxybenzoic acid, 2. Catechin, 3. Caffeic acid, 4. Chlorogenic acid, 5. P-coumaric acid, 6. Resveratrol, 7. Myricetin.

Figure 2. Free radical scavenging activity of red grapes varieties extracts measured by the DPPH assay.
in the scavenging activity of the grape phenolic extracts, Table 6 presents the flavonoid and non-flavonoid contents of grape extracts with the inhibition of the free radical DPPH. A correlation between the flavonoid content and the inhibition of the DPPH radical was noticed in Table 6. In fact, it seems to be clear that the basic structure of compounds and other structural factors are very important in the scavenging mechanism [41]. Table 6 showed that the scavenging effects of red grapes on DPPH radicals increased when the flavonoid concentration increase. This result showed that flavonoid are the main responsible of the scavenging activity of red grape extracts, which was also proved by many studies [42,43]. Earlier studies indicated that the ability of flavonoids to incactivate peroxyl radicals was in the main better than the small phenolic antioxidants [44]. Other approaches also have established that the position and degree of hydroxylation is fundamental to the antioxidant activity of flavonoids [45].
Moreover, Table 6 showed significant differences between scavenging activities of the different grapes cultivars. Cabernet sauvignon grapes extract and merlot have the greatest activity toward DPPH radical, but there were not the extracts with the highest phenolic content. Many authors confirmed that there is an insignificant correlation between free radical scavenging capacity and phenolic content, suggesting the presence of further phenolic components or interactions involved in the antioxidant potential [46].
In fact, in the phenolic pool of red grapes, there are some secondary compounds that are important for their antioxidant activity: Catechin and Epicatechin (flavan- 3-ols), Quercetin [47,48] and its glycoside Rutin (flavonols), and trans-Resveratrol (stilbene). These compounds have been proven to be potent antioxidants and to have important biological, pharmacological and medicinal properties [49]. In our study, TACS22 and KAM33 represent the greatest scavenging activity due to their high concentration of these compounds. Theses extracts contain most of the flavonoids components identified by HPLC (Tables 3 and 4). A synergistic effect of these various phenolic compounds could explain the high scavenging activity of the TACS22 and KAM33 extracts. This effect was also discussed by Sun and Ho, 2005 [36] who proved that the synergistic effect of the antioxidants in the extracts should also be considered.
The Cabernet franc extract represents the lowest scavenging capacity, due to the absence of Quercetin and Catechin in this extract.
After studying the scavenging activity results, an antimicrobial studies on the efficacy of the phenolic compounds of Lebanese red grapes extracts to inactivate the bacterial and fungal strains should be discussed.

Table 6. Flavonoid, non-flavonoid and % of inhibition of the extracts.
3.3. Antimicrobial Assays
Due to the development of resistant microbial strains, the number of publications on antimicrobial activity of phenolic compound is increasing. The two most commonly used methods for the screening of the potential antimicrobial plant compounds were the disc diffusion test and the dilution plate assay. These techniques do not distinguish bactericidal and bacteriostatic effects and permit to determine just an approximate minimum inhibitory concentration (MIC) [50]. Recently, the microplate method was used to the screening of antimicrobial compounds. It provided a potentially useful technique for determining MICs of large numbers of test samples. It consisted on adding phenolic extract on the well of an ELISA tray filled with the exponentially growing culture (about 108 colony-forming units/ml). The plate was incubated at 37˚C for 18 h, agitated and the absorbance read after the incubation were subtracted of those read before, at the same wavelength (620 nm). The application of this method was not adequate on the grape extract. This is due to the fact that the red grape extract and the inoculum of bacteria read at the same absorbance which induces an interference of the color of the tested substance with the bacteria. In order to avoid this problem, a new quantitative method was adapted in our study to determine the antimicrobial efficacy of the Lebanese red grape extracts. It consists on counting the numbers of bacteria or yeast colonies that grow on each plate after the addition of the phenolic extract on the plate containing the bacterial or fungal culture.
Antimicrobial activities of antimicrobials agents (Chloramphenicol), phenolic standards (Resveratrol, BHT, Gallic acid) used as positive controls (at a concentration of 5 mg/mL) and red grape extracts against Gram-negative strains (S. arizonae and E. coli), Gram-positive strain (L. monocytogenes) and a fungal strain (C. albicans) were studied. The antimicrobial activity toward S. arizonae and E. coli (Gram-negative strains) is presented is Figure 3.
Figure 3 showed that Chloramphenicol presented the highest growth inhibition on Gram-negative strains, and that natural phenolic compounds has more effect than