The Relationship between Antioxidant and Anti-Ulcer Activities in Saudi Honey Samples Harvested from Various Regions in Different Seasons ()
1. Introduction
Honey, which is used in both domestic and medicinal applications, has been widely used as a sweetener since ancient times. The composition of honey varies depending on the geographical and nectar sources of a region. The quality of honey depends on its physio-chemical and sensory properties. Hence, knowledge about its constituents is essential for assessing its quality [1,2].
Certain phenolic acids and flavonoids are described in the literature as marker substances for several unifloral honeys [3]. Because the methods for extraction and determination have varied among studies, 37 phenolic acids and flavonoids are described in the literature [3,4].
Ulcerative lesions of the gastrointestinal tract are one of the major side effects associated with alcohol consumption [5]. Gastric ulcers are benign lesions occurring at a site where the mucosal epithelium is exposed to acid, alcohol, and pepsin. Several factors are implicated in the pathogenesis of gastric ulcers including stress, smoking, nutritional deficiencies and ingestion of non-steroidal antiinflammatory drugs [6,7]. In recent years, a powerful association between gastric ulcers and infection of Helicobacter pylori has been adopted [8]. Gastrointestinal bleeding is the most common complication of ulcers. A sudden loss of a large amount of blood can be life-threatening, and occurs when the ulcer erodes a blood vessel. Scarring and swelling caused by ulcers may obstruct the gastric outlet.
Numerous products are used for treating gastric ulcers including H2-blockers, proton pump inhibitors that reduce acid secretion, and sucralfate, which provides mucosal protection. Although these drugs have changed ulcer therapy remarkably, their efficacy is still debatable. Reports on clinical evaluations of these drugs show that there are incidences of different adverse effects and drug interactions during ulcer therapy [9]. Thus, screening plants and other natural compounds for active drugs is still critical and might provide a useful source of new anti-ulcer products for developing pharmaceutical drugs or, alternatively, simple dietary adjuncts to existing therapies [10].
Therefore, this study assessed the efficacy of honey samples for their gastro-protective effects in rats, as well as the study of antioxidant, and conducted phytochemical and chromatographic analysis to determine their main chemical components.
2. Material and Methods
2.1. Honey Samples and Identification
Thirteen honey samples were collected from beekeepers and divided into three main groups (summer, winter, and both). None of the samples showed signs of fermentation or granulation. Table 1 lists the 13 honey samples.
2.2. Animals
Male albino rats (220 - 230 g) and mice of both sexes (25 - 30 g) were used. The rats were maintained in cages with raised floors of wide wire mesh to prevent coprophagy, whereas the mice were housed randomly in groups in polypropylene cages. All of the animals were kept under uniform and controlled conditions of temperature and light/dark (12 h/12 h) cycles. The animals were fed a standard pellet diet and water ad libitum and were left for one week to acclimatize to the conditions of the room.
2.3. HPLC Analysis
2.3.1. For Amino Acids
1) Standards and Sample Preparation Standards of amino acids (Sigma, St. Louis, MO) and our honey samples were prepared as described by A. Fabiani, et al. [11]. Identification was based on comparing the retention times of the standards amino acids with those in the honey samples and was confirmed by performing a fortification technique.
2.3.2. For Flavonoids
To identify the flavonoids, an Agilent 1100 HPLC apparatus was used. In the high-performance liquid chromatography (HPLC) analysis, a Zorbax SB-C18 column 250 mm in length, 4.6 mm i.d., and 5 μmin particle diameter was used. To detect flavonoids, a wavelength of 337 nm was set. The mobile phase was acetonitrile-water at a ratio of 48:52(v/v); the temperature was 25˚C, with a flow rateof 0.3 ml/min; and the injected sample volume was 20 μl. Diluted standard solutions of rutin, quercetin, apigenin, kaempferol, acacetin, chrysin, myricetin, luteolin, tricetin, cinnamic acid, gallic acid and caffeic acid were analyzed under the same HPLC conditions. Furthermore, the detector response was calibrated, and the honey samples were dissolved in 0.5 gm/100 ml of deionized water.
2.3.3. For Carbohydrates
A hyopercarb column, 5U 100 × 4.6 mm in length was
used, and the detector was RI detector, pump was GBC LC 1110 pump, software Winchrome Chromatography Ver.13, and the flow rate was 1 ml/min. The optimalmobile phase was a mixture of acetonitrile and water in the ratio of 85:15 (v/v).
Standard sugars were obtained from Sigma (St. Louis, MO). Standard solutions of sugars made up by as solving 5 g of each sugar in 1000 ML of distilled water. The honey samples were diluted in water prior to analysis (0.5 g/100 ml H2O). All standards and samples were filtered and degassed by using vacuum filtration. Before injection onto the column, all syringes were fitted with syringe filters. The retention times for all standards were noted and tabulated.
2.4. Antioxidative Activity
The honey samples were analyzed for antioxidant activity by using the DPPH method. The reaction of DPPH with the honey samples was measured spectrophotometrically, at 517 nm on a Perkin Elmer, Lambda EZ Series spectrophotometer, and the data were analyzed using the corresponding UV-Vis Analyst ver. 4.67. The crude honey diluted samples, and tenth fold, were mixed with 1 mM of a DPPH ethanolic solution in 96% ethanol, and the absorbance at 517 nm was measured (the ratio of the honey sample, DPPH solution, and ethanol were 1:1:13). The antioxidative activity of the honey sample was evaluated as the relative absorbance of the sample in the presence of the DPPH solution after the constancy of the absorbance at 517 nm by using the following formula:
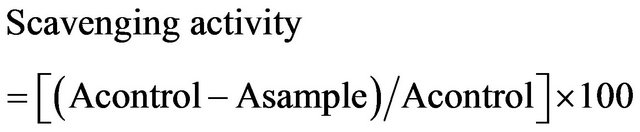
where A is the absorbtion at 517 nm [4].
2.5. Ulcerogenic Activity
2.5.1. Preparation of Honey Samples
A known weight of each of honey sample and the reference drug (ranitidine) was dissolved in 3% v/v Tween 80 to produce solutions of 10% concentration. The ulcerogenic agent, ethanol (Merck), was diluted to 50% (v/v, in distilled water).
2.5.2. Doses
In the absence of LD50 values for the honey samples, an experimental dose of 100 mg·kg−1 was selected in this investigation to be administered orally to rats. The reference drug; ranitidine was administered orally at a certain dose (30 mg·kg−1). This dose was calculated by converting the therapeutic dose used in human to rat’s dose according to the findings of Paget and Barnes [11]. Ethanol (50%) was administered orally to the rats ata dose of 10 mL·kg−1.
3. Phytochemical Analysis
3.1. Phytochemical Screening
The preliminary phytochemical screening of the 13 honey samples indicated the presence of phenolic compounds (flavonoids, phenolic acids, and coumarins.), amino acids, and carbohydrates. One percent of each successive ethyl acetate and water extracts were chromatographed on pre-coated silica gel plates by using the following solvent systems:
a—Chloroform—Methanol (95:5 v/v.).
b—Ethyl acetate—Methanol—Water (30:5:4 v/v/v.).
c—Benzene—Ethyl acetate (80:20 v/v/).
Solvent systems a and b achieved the most effectiveseparation, and the chromatograms from these systems were visualized by using UV lamp (short and long wave length) before and after spraying with AlCl3, (a spray reagent for detecting flavonoids). The presence of amino acids was determined by spraying with ninhydrin on TLC plates.
3.2. Amino Acids
Sixteen amino acids or related compounds were identified in the honey samples by using HPLC. Proline, aspartine, threonine, serine, glutamine, glycine, alanine, valine, isoleucine, leucine, tyrocine, phenylalanine, lycine, argenine, and histidine, in addition to aspartic acid and glutamic acid, were found in all of the honey samples (Table 2).
3.3. Flavonoids
The flavonoids and phenolic acids of honey samples from different seasons and geographical regions were analyzed by using HPLC. More than eight flavonoids were identified in honey (Table 3), with an average content of 6.68 mg/100 g of honey. Luteolin (5,7,3’,4’-tetrahydroxy flavone) tricetin (5,7,3’,4’,5’-pentahydroxy flavone), apigenin, chrysin (5,7-dihydroxyflavone),and myricetin (3,5,7,3’,4’,5’-hexahydroxy flavone) were the main flavonoids identified in summer honeys, whereas quercetin (3,5,7,3’,4’-pentahydroxy flavone), isorhamnetin (3,5,7,4’- tetrahydroxy flavone 3’-methyl ethyl), ruten, and kaempferol were the main flavonoids identified in winter honey. The mean content of total phenolic acids in both honey samples was arranged from 3.14 - 4.34 mg/100 g of honey, with gallic, caffic, and coumaric acids determined to be the potential phenolic acids. The content of total phenolic acids was as much as 10.08 mg/100 g of honey, with gallic acid as the main component.
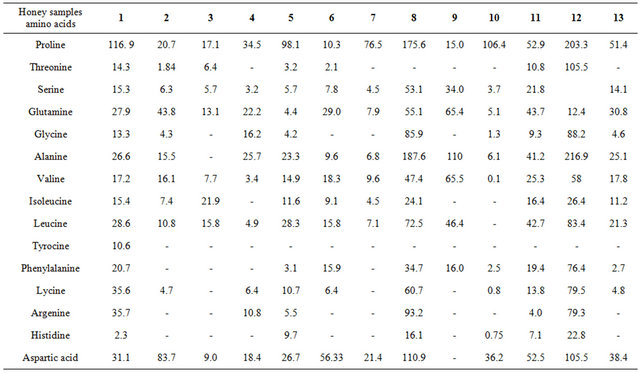
Table 2. HPLC analysis of amino acids in 13 honey samples.

Table 3. Systematic names of the standard flavonoids used in this study.
3.4. Carbohydrates
Table 4 lists the results obtained from performing HPLC analysis to determine the carbohydrate content in the 13 honey samples. In all of the honey samples, 5 carbohydrates were evaluated and quantified: monosaccharides (fructose, glucose, arabinose, and galactose) and disaccharides (maltose). Regarding the galactose contents in all of the 13 honey samples, three of the samples (Samples 3, 5, and 8) had a higher galactose content than the others did. Significant differences were found for the carbohydrate content in the honey samples. The main distinguisher for these products was fructose content (found only in Sample 11, 0.06 mg/1 ml of honey) and high galactose content (more than 2.58 mg/1 ml of honey). The lowest glucose values were found in Sample 7 honey (averaging), whereas the average value for the other samples was 0.22 mg/1 ml.