Synthesis, characterization of a multi-component metal oxide (Al0.88Fe0.67Zn0.28O3) and elimination of As (III) from aqueous solution. ()
1. INTRODUCTION
Arsenic is one of the most widely spread element in the earth’s crust and biosphere [1]. It is one of the most hormone disruptors of body blood stream and is defined as one of the most poisonous and carcinogenic [2] chemical element. It is a group—A human carcinogen according to US-EPA [1]. The presence of arsenic in ground water and eventually in drinking water [3] causes a serious environmental threat due to its toxicity. Because of harmful affect of arsenic, the removal of trace amount of arsenic from industrial effluents has been an important area of study. The most common form of arsenic [4] species present in drinking water as As (V) and As (III). As (III) is considered more toxic than arsenate [2] and tends to be more mobile in the environment. The World Health Organization (WHO) [5] and Bangladesh [6] have established a provisional guideline of 10 ppb and 50 ppb for arsenic in drinking water respectably. Arsenic concentration value was found at higher level than WHO recommended value which is more than 50% [7] of the tube-wells in Bangladesh. Around 40 million people [8] are at risk for ground water arsenic contamination and this figure is increasing day by day. Though already several technologies [9] have been established but it is very difficult to select a unique method for arsenic removal. Some of them are effective but economically impracticable. On the other hand, some are user unfriendly, technologically unsound, energy or skilled manpower dependent. Sometimes quality of treated water does not maintain standard parameters of drinking water. Even specific information on the major factors affecting arsenic removal is also still incomplete. For this reason, effective technology development for arsenic removal is a necessity. Usual treatment for arsenic removal includes coagulation-precipitation using iron and aluminum substances, ion exchange, reverse osmosis, nanofiltration, bioremediation and adsorption [10-14]. Metal oxide materials have good affinity to arsenic [9]. Multi-metal-oxides are of major commercial importance. With different morphologies and sizes, oxide materials can bring various potential applications into many fields including catalysis and adsorption [9]. To predict the structures for these complex oxides both under equilibrium conditions at the temperatures and pressures of interest and the dynamical structures as the reactions proceed is an important area of study. Investigating the mechanism for the multi-metal-oxides composites and to seek ways to improve the selectivity for interacting with selective species is a necessity. The dominant As ionic species in the aquatic environment are As (III) (H2AsO3–) or As(V) (HAsO42–) depending upon pH. Arsenic may retained in composite metal oxides by adsorption on surface of iron zinc and aluminum oxides and hydroxides as well as by ligand exchange [15]. Arsenic mobility in the environment is dependent on its interaction with metal oxides. Binding of arsenic to different solid phases via surface complexation is well documented in the literature [16,17]. Arsenic species binds strongly with the metal oxides of Fe, Al and Mn. However, the binding mechanisms are dependent on the pH and redox potential of the environment [18]. Surface complexation reactions on metal oxides affect the fate and the transport of arsenic in environmental systems and the global cycle of this element. The substitution of Fe3+ ions by Al3+ ions in the solid surface has been observed, indicating an alternative removal mechanism of arsenic in these metal hydroxides and oxyhydroxides by providing larger surface area for arsenic adsorption via retarding the crystalline formation of iron [19]. Our aim was to synthesize Multi-Component Oxide (Al0.88Fe0.67Zn0.28O3) surface abbreviated as MCOS which were increased the degree of elimination of As (III) from aqueous solution. There is a variety of synthesis methods for the preparation of metal oxide such as co-precipitation, sol-gel, and hydro-thermal [20]. In the present study a new MCOS, Al0.88Fe0.67Zn0.28O3 was successfully synthesized using co-precipitation method for its simplicity.
2. MATERIALS AND METHODS
Al(NO3)3·9H2O (BDH, England), Fe(NO3)3·9H2O (BDH, England), ZnSO4·7H2O (E. Merck, Germany) and Na2CO3 (BDH, England) was utilized to prepare the MCOS. MCOS was prepared by co-precipitation method. The MCOS sorbent was characterized by X-ray Diffraction (XRD), Scanning Electron Microscopy (SEM), Energy Dispersive X-ray Spectroscopy (EDXS), X-ray Fluorescence Spectroscopy (XRF), Thermogravimetry-Differential Thermal Analysis (TGA-DTA), Differential Scanning Calorimetry (DSC). Determination of specific surface area of MCOS was performed by using Methylene Blue (E. Merck, Germany) dye. The As (III) solution was prepared by As2O3 (BDH, England) and NaOH (Merck, India). All solution preparation through this research work was conducted by triple distilled water. All chemicals were analytical grade and used without a further purification
3. EXPERIMENTAL
3.1. Preparation of MCOS
MCOS was prepared by co-precipitation of their carbonates from the aqueous solution of the metal nitrates and sulfates. Required concentration of solutions of Al(NO3)3·9H2O, Fe(NO3)3·9H2O, ZnSO4·7H2O and Na2CO3 in triple distilled water were prepared. First of all, Al(NO3)3·9H2O, Fe(NO3)3·9H2O and ZnSO4·7H2O solutions were mixed together in a beaker in 1:1:1 ratio and stirred (STUART Scientific Magnetic Stirrer and Hotplate) vigorously at room temperature for 30 minutes. Then the resultant solution was heated slowly using Bunsen burner at about 70˚C - 80˚C. Then the solution of Na2CO3 was added slowly, until precipitation was complete. The precipitate was separated by suction pump and was washed thoroughly with distilled water. The washed sample was transferred to a Petri dish and taken in an oven (Model 0255 Gen Lab, England). The temperature was raised to 90˚C and kept at that temperature for 2 hours. Then the solvent was dried at 130˚C for 3 hours. The dried sample was then calcined in air in a Muffle Furnace (6000 Thermolyne, England) for 6 hours at 400˚C. The calcinations converted the carbonate of the sample into their respective oxides
3.2. Characterization of MCOS
The MCOS sorbent was characterized by X-ray Diffraction (XRD) using a PANalytical (Netherland) X-ray Diffractometer equipped with Cu Ka radiation (λ = 1.54187 Å) at a step width of 0.02˚ operated at 40 kV and 30 mA. TGA and DSC of MCOS were performed using Q-50 (USA) and Q-10 (USA) max. Working temperatures were 800˚C and 600˚C respectively and was worked at 20˚C/min. For this experiment, 19.6910 mg and 10.00 mg samples were taken respectively to study the weight loss and rate of heat flow pattern of MCOS with temperature. Compositional analysis was carried out using Rigaku (Japan) ZSX-primus, at 50 kV and 40 mA. The surface morphology was shown by Scanning Electron Microscope (SEM). Elemental analysis was also performed by Energy Dispersive X-ray Spectroscopy (Philips XL30, USA, Acc. Volt. 30 kV).
3.3. Preparation of As (III) Solution
As (III) stock solution was prepared by dissolving As2O3 and NaOH in triple distilled water. The required solutions were prepared using standard pre-prepared stock solution.
3.4. Batch Experiments
In 100 mL known concentration of As (III) solution was added required amount of MCOS. The solution was stirred by magnetic stirred (MM 2A, LABORATRNI PRISTROJEPRAH) for required time at room and different temperature. After that the solution was centrifuged and filtered. Finally As (III) concentration of the filtrate was determined. Difference between two data showed the amount sorbed by the MCOS. The pH of the solution was adjusted by NaOH and HCl (Merck, India).
3.5. Method Used for Measurement of As (III) Concentration
In this research work, measurement of As (III) concentration was carried out by Digital Arsenator (WAG-WE 105000), which provided a digital read-out of arsenic concentration. The arsenic detection limit of Digital Arsenator is 0 to 100 ppb. In dilution method higher concentration of arsenic is also detectable. Arsenetor has been used for arsenic testing in Bangladesh and Myanmar [21]. As a field based instrument, it was evaluated in Myanmar and gave an above average consistency [22]. The Pearson correlation between the silver diethyldithiocarbamate method and the arsenator was found to be 0.87 for arsenic concentrations in the range 0 - 100 μg/L [23]. The overall performance of the arsenator was rated as excellent by the Shri Ram Institute for Industrial Research [24]. The method had been used by the British Geological Survey (BGS) alongside samples later analyzed by AAS, with a good concordance recorded [25].
4. RESULTS AND DISCUSSION
4.1. Characterization of MCOS
Figures 1 and 2 show the XRD pattern of metal composite oxides. The major peak was found at 2θ = 44.6489˚ which matches the standard file of 99Al2O3·ZnO and peak comes from (211) plane (JCPDS card no. 22 - 1034). We found another peak from (400) and (622)
plane of Fe2O3 (JCPDS card no. 32 - 469), which indicate the presence of Fe2O3. Peak from (533) plane of Fe3O4 was found as well. Hence, from XRD analysis we can claim that MCOS of Al0.88Fe0.67Zn0.28O3 was synthesized. This was also confirmed by elemental analysis of EDXS (Energy Dispersive X-ray Spectroscopy) and XRF. From EDX spectroscopy as shown in Figure 3, we can observe that Al, Fe and Zn concentration peak were found at around 1.485 keV, 6.398 keV and 8.628 keV energy respectively as well as oxygen concentration peak was also found below 1 keV energy. The larger peaks came from K shell energy and some of them from M shell and L shell. According to the EDXS, the average quantities of Al, Fe, Zn and O in the prepared MCOS are 18.2%, 28.80%, 14.15% and 36.9% where as from XRF analysis we found oxides of Al, Fe and Zn were 23.6%, 39.9% and 20.6%. The surface morphology of MCOS for 1000X in Figure 4 is exhibited by SEM image. The size of the MCOS particles are found in μm level, 10 - 20 μm. DTA-TGA and DSC of MCOS provide information about the trend of weight loss and heat flow with increasing temperature up to 800˚C for DTA-TGA in Figure 5 and up to 600˚C for DSC in Figure 6. As shown in Figure 5 DTA shows peaks at 57 and 117˚C corresponding to a weight loss in TGA and a peak at 722˚C corresponding to another weight loss in TGA. The DSC thermogramme is characterized by a strong endothermic peak at 132˚C and small peaks at 95, 282 and 362.5˚C as shown in Figure 6. Unfortunately, we did not get clear information of MCOS for decomposition reaction since weight loss was only near about 20%. The

Figure 4. SEM image of MCOS (Meg. 1000X).

Figure 5. TGA-DTA Analysis of MCOS at temperature 800˚C.

Figure 6. Differential Scanning Calorimetry (DSC) of MCOS at temperature 600˚C.
reason is that individual melting temperature of the Aluminum oxide, Iron oxide and Zinc oxide is higher than our experimental temperature. Using Langmuir and Hinshelwood model the calculated specific surface area of MCOS was measured and found to be 389.85 m2·g–1.
4.2. Sorption Studies of As (III) from Synthetic Aqueous Solution by MCOS
Batch experiments were performed to remove As (III) from aqueous solution taking various parameters such as effect of pH, contact time, initial arsenic concentration, temperature and sorbent dosage. The effect of pH on the process is listed in Table 1. The data illustrated that the removal of As (III) by MCOS was almost steady from pH 4 to 9. The World Health Organization (WHO) recommended range of pH is 6.5 to 9.2 [26]. It is anticipated that MCOS is effective to eliminate As (III) from drinking water without any difficulties.
Sorption kinetics experiments were carried out at room temperature to find the equilibrium times for As (III) sorption onto MCOS. It has been showed that sorption of As (III) is time dependent becoming greater with increasing time but rapid during initial stage and decreases when approaching equilibrium. An adequate pseudo-second order rate constant k for the sorption was determined using a linear plot of t/qt versus t by following equation [27].
The theoretical amount sorbed at equilibrium qeq also calculated using an Equation 1.
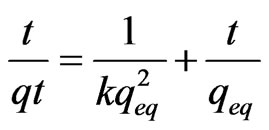 (1)
(1)
where, t represent the time, qt is amount sorbed at time t. The plot of t/qt versus t shows a straight line in Figure 7. The regression coefficient is close to 1 (0.99) which indicates the sorption is said to follow pseudo-second order kinetics. The pseudo-second order rate constant k and theoretical amount sorbed at equilibrium for As (III) sorption on MCOS is calculated to be 80 × 10–2 g·mg–1·min–1 and 138 × 10–3 mg·g–1 at 25˚C ± 3˚C.
The sorption capacity of As (III) from aqueous solution by MCOS were performed at various temperature. The employed temperatures were 298, 313, 333 and 353 K. The equilibrium partition constant kd is interpreted by following Equation 2.
 (2)
(2)
where, qeq is the amount sorbed by sorbent and Ceq is equilibrium concentration.
The thermodynamic parameters such as ∆G˚, ∆H˚ and ∆S˚ are assessed by using following equation [28-30].
 (3)
(3)
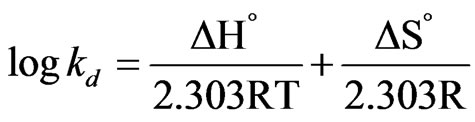 (4)
(4)
The negative values of (∆G˚) denotes the sorption of As (III) from aqueous solution on the MCOS is spontaneous, conceivably owing to columbic attraction [27]. The value of ∆H˚ and ∆S˚ are found from the plot of log Kd Versus 1/T which is shown in Figure 8. The value of ∆H˚ is positive. For ion exchange process, the sorption energy should be within the range of 8 - 16 kJ·mol–1. The sorption energy is 9.38 kJ which indicates that the sorption may be ion exchange process. The negative values of ∆S˚ (–22 J·mol–1) are sign of the affinity [30] of the MCOS towards As (III).
The equilibrium data is compare to evaluate the performance of MCOS sorbent. In this study, Langmuir and Frenundlich model have interpreted for using equilibrium data. The linear form of the Langmuir and Frenundlich model is expressed in the following Equation 5 and 6 respectively.
 (5)
(5)
 (6)
(6)
where Ceq is the equilibrium As (III) concentration, qeq is the amount absorbed at equilibrium, qm is the maximum sorption capacity at mono layer coverage and KL, KF and n are isotherm constant.
Throughout this study, batch experiment data is found to give good correlation coefficient (0.98) with Langmuir than Frenundlich isotherm. The important assumption for the application of the Langmuir isotherm are 1) Homogenous surface site, 2) Monolayer sorption on a surface containing a finite number of identical sites and 3) Uniform surface energies of sorption [30]. The numerical values of the Langmuir constant (qm and KL) are found from the plot of Ceq/qeq versus Ceq (Figure 9). The maximum sorption capacity of MCOS at monolayer (qm) and equilibrium constant KL is calculated as 13 × 10–2 mg·g–1and 9.1 × 10–3 L·mg–1.
The dependence of As (III) sorption on MCOS dosages with varying amount have been studied which are shown in Table 2.
Elimination of As (III) from aqueous solution is strongly dependent on the MCOS dosages which indicates the sorption is dependent on the availability of bonding sites. But the magnitude of the increase is proportionally less significant with each successive increase. The interesting point is noted here that after certain period of time (60 min), 410 ppb As (III) was completely removed at one gram dosage of MCOS maintaining the pH 8 and temperature 28 ± 3˚C. The sorption of As (III)
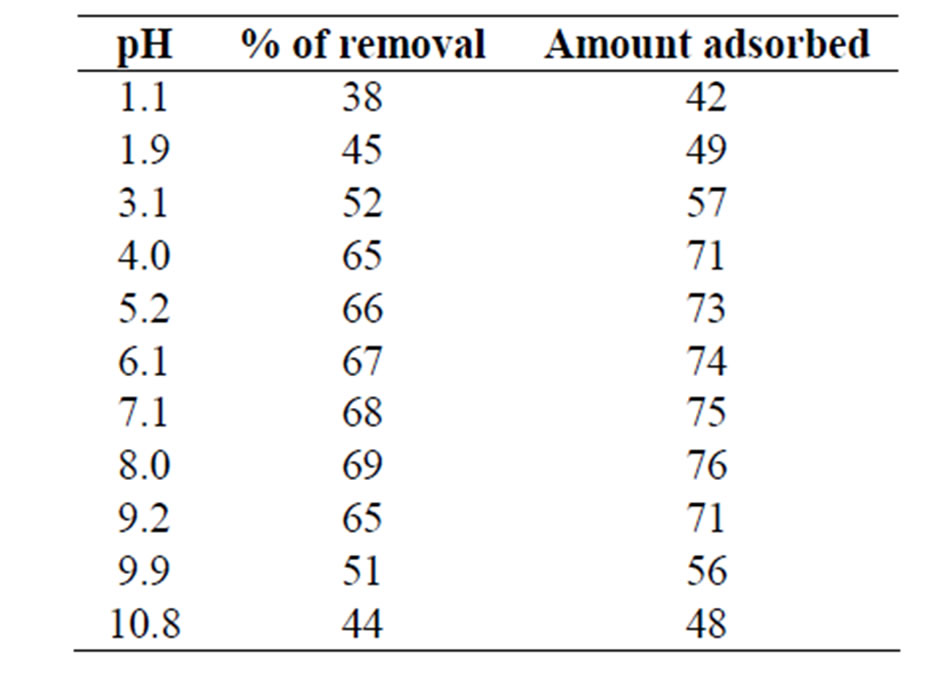
Table 1. Effect of pH on As (III) sorption by MCOS. As (III): 100 mL, 440 ppb, MCOS: 0.4 g, Temperature: 28 ± 3˚C, Contact time: 120 minutes.

Figure 7. Second order kinetics for sorption of As (III) by MCOS. As (III): 100 mL, 410 ppb, MCOS: 0.4 g, pH: 8 ± 0.2, Temperature: 28 ± 3˚C.

Figure 8. Van’t hoff plot for the sorption As (III) by MCOS. As (III): 100 mL, 410 ppb, MCOS: 0.4 g, pH: 8 ± 0.2, Contact time: 60 minutes.

Figure 9. Langmuir plot for the sorption of As (III) by MCOS: 100 mL As (III), MCOS: 0.4 g, pH: 8 ± 0.2, Temperature: 28 ± 3˚C, Contact time: 210 minutes.

Table 2. Data for the effect of MCOS dosages on sorption of As (III).
from aqueous solution on the MCOS is spontaneous owing to columbic attraction. As shown in Table 1 the removal of As (III) by MCOS was almost steady from pH 4 to 9 showing maximum removal at pH 7 to 8. Surface ionization (protonation and deprotonation) reactions take place depending on the pH of the solution in contact with the solid. The pH dependence of As adsorption for solids is usually explained in terms of ionization of both adsorbates, As and adsorbents (MCOS). At pH values corresponding to natural pH of water, MCOS is able to adsorb more than 70% of arsenic. The binding mechanisms are thus dependent on the pH and redox potential as well as the surface area of the MCOS system. The specific surface area of MCOS found to be 389.85 m2·g–1, which is much larger than that of iron oxides (30 - 273 m2·g–1) facilitating the strong adsorption of As on composite [15]. Multi metal oxide, Al0.88Fe0.67Zn0.28O3 due to its high surface area play important role in As retention because arsenic species adsorbs strongly to metal-oxide surfaces. Metal oxides sorbs arsenic through ligand exchange mechanism. Most arsenate and arsenite oxyanions replaced singly coordinated surface OH groups to form bridging complexes like M-O-AsO(OH)-O-M and M-O-As(OH)-O-M at high surface coverage. At a very low surface coverage, monodentate complexes may also form on oxides. Complexation remains the major bonding mechanism for arsenic adsorption on oxides as well as ligand exchange. Mixed valent iron media as found from XRD analysis, with ZnO and Al2O3 may also play role in the mechanism for arsenic adsorption on Al0.88Fe0.67Zn0.28O3 [31].
5. CONCLUSIONS
The composite oxide material was developed to remove As (III) from aqueous solution and characterized by XRD, SEM and EDXS. The sorptive ability of MCOS for As (III) is excellent. The sorption capacity of MCOS at monolayer (qm) and equilibrium constant KL are 13 × 10–2 mg·g–1 and 9.1 × 10–3 L·mg–1 respectively. The negative values of (∆G˚) indicates the sorption of As (III) from aqueous solution on the MCOS is spontaneous owing to columbic attraction. It may effective as adsorbent because of its easy and simple synthetic way. The kinetic, thermodynamic and equilibrium study were performed. At pH values corresponding to natural pH of water, MCOS is able to adsorb more than 70% of arsenic. The binding mechanisms are dependent on the pH and redox potential as well as large surface area of the MCOS system, Al0.88Fe0.67Zn0.28O3. To better understand the sorption mechanism of MCOS further study will be needed. Study using naturally contaminated water with arsenic is underway, which will be subject matter of our subsequent paper.