1. Introduction
Magnetic nanoparticles (MNPs) coated with temperaturesensitive polymers have been given great attention because of their various applications in the fields of biotechnology and medicine. Especially, temperature-sensitive coated MNPs have been used comprehensively in controlled and targeted drug release systems [1-3]. These nanoparicales are better to the traditional stimuli-responsive systems such as pH and temperature-sensitive polymers, because they offer the benefit of noncontact force (e.g., an external magnetic field) [4,5]. The external magnetic field is used to guide nanoparticales to a disease site and persuade heat as a motivation to the polymer shell [5,6]. These magnetic targeted carriers have also been designed with dual functionality as imaging agents and drug carriers [7]. In general, these systems are capable of site-specific targeting and controlled and sustained drug release with high biocompatibility because of the reduction in systemic toxicity [8,9].
One of these temperature-sensitive polymer-coated MNPs, poly-(N-isopropylacrylamide) (PNIPAAm)-coated MNPs are given particular attention because of their stimuli (temperature) responsiveness and better drug-loading ability [10,11]. These characteristics are due to amphiphilicity, their large inner volume, capacity for manipulation of permeability, and response to an external temperature stimulus with an on-off mechanism [12].
However, one potential problem with using PNIPAAm as a polymer coat is that its lower critical solution temperature (LCST), the temperature at which a phase transition occurs, is lower than body temperature (32˚C). To increase the LCST of PNIPAAm above body temperature, it has been co-polymerized with different monomers, such as acrylamide (AAm) [13]. To increase the site-specific targeted capability of PNIPAAm-AAm, it is necessary to incorporate monomers consisting of functional groups such as amine for conjugation of antibodies specific for target cells. Functionalization of the polymer would introduce impurities and change the LCST dramatically. Therefore, there is a need to suitably functionalize the nanoparticles without changing the LCST [14,15].
In this research, we have developed a process for covalently coating MNPs with PNIPAAm and P(NIPAAmMAA-VP). We have shown that these PNIPAAm-MAAVP-coated MNPs have a LCST above body temperature and functional groups on their surface for conjugation of biomolecules [16]. Also we intend to investigate the in vitro characteristics of our nanoparticles for drug delivery applications. To manufacture the PNIPAAm-MAAVP-coated MNPs, NIPA, MAA, and VP were then polymerized the MNPs via methylene-bis-acrylamide and benzoil peroxid (BP) as a cross-linking agent and an initiator, respectively. The nanoparticle size and morphology were analyzed using scanning electron microscopy (SEM). The drug release behavior of doxorubicin DOX, an anticancer drug model from the nanoparticles at temperatures below and at the LCST was also analyzed. Furthermore, it would be possible to image the cancer in vivo and discern the effect of the therapy on the tumor [17]. This type of multifunctionality (ability to image and provide therapy) in MNPs has recently been gained interest [18].
2. Experimental Procedures
2.1. Materials and Methods
Ferric chloride hexahydrate and ferrous chloride tetrahydrate were purchased sigma Chemical co (USA). Methacrylic acid (MAA), N-isopropylacrilamid (NIPPAMs), ammoniumhydroxide, methylene-bisacry-lamide (BIS), doxorubicin (DOX), polyvinilalch (PAV, MW = 27000), benzoil peroxide (BP),1,4 dioxan were purchased from Aldrich and used as received.
2.2. Synthesis of Poly (NIPAAm-MAA-VP)
Poly (NIPAAm-MAA-VP) nanoparticles of three different ratio (according to table 1) were synthesized by free radical polymerization reaction. NIPAAM, VP, MAA, and BIS (as the cross-linking agent were) dissolved in 20 mL of 1,4-dioxan in to which 0.3 mol % BP with respect to all the monomers was added. The mixture was magnetically stirred and degassed with nitrogen for 30 min. The polymerization was carried out at 70˚C for 24 h with continuous nitrogen bubbling and the polymer was obtained by precipitation in an excess amount of cold n-Hexan.
2.3. Synthesis of Fe3O4 Nanoparticles
The preparation of Fe3O4 nanoparticles was followed by a chemical co-precipitation of Fe2+ and Fe3+ ions descrybed previously [19]. With some modifications, 28 mmol FeCl2 and 16 mmol FeCl3 were prepared in 50 mlit deionized water in two beakers, and then transferred to a 250 ml three-necked flask together, stirred under nitrogen (Figure 1). When the solution was heated to 60˚C, NH3-H2O (25 wt%) was added drop wise until pH 10 - 11. After base was added, the solution immediately became dark brown, which indicates iron oxide has been formed in the system. The solution was heated at 80˚C for 1 h. The precipitates were isolated from the solvent by magnetic decantation and repeatedly washed with deionized water until neutral, then were dried at room temperature under vacuum for 12 h.

table 1 . Conditions used for preparation of P (NIPAAmMAA-VP).

Figure 1. Reactor and the reaction equation of preparation magnetic Fe3O4 nanoparticle.
2.4. Immobilization of P (NIPAAm-AAm-VP) on the Surface of MNPs
MNPs were used as a template to polymerize in an aqueous micellar solution. BIS was as cross-linking agent, as previously described with a small modification [20]. In brief, 0.028 g of MNPs, 0.1 g of NIPA, 0.0129 g of AAm, 0.0345 mL of VP, 0.0131 g of BIS, were sonicated in 100 mL cold water for 30 minutes. Then, 0.078 g of ammonium persulfate was added to the solution, and the reaction was carried out at room temperature under nitrogen gas for 4 hours. The product was purified several times with DI water by using a magnet to collect only PNIPAAm-VP-coated MNPs. PNIPAAm-MAA-coated MNPs was also formulated using the same synthesis process for deferent ratio of materials.
2.5. Drug Loading
For drug-loading and drug release studies, DOX was used as a model drug. In brief, 60 mg of freeze-dried PNIPAAm-MAA-VP, MNPs 5 mg and 12 mg of DOX were dispersed in phosphate buffer solution (PBS). The solution was stirred at 4˚C for 3 days. The DOX-loaded PNIPAAm-MAA-VP-coated MNPs were separated from the solution using an external magnet. The solution was then analyzed by using an ultraviolet-visible (UV-Vis) spectrofluorometer (Infinite M200 plate reader; Tecan, Durham, North Carolina) to determine the amount of unencapsulated DOX (λex 483 nm). This value was then compared to the total amount of added DOX to determine the DOX-loading efficiency of the nanoparticles. Loading efficiency was calculated according to the following formula:

2.6. In Vitro Drug Release Kinetics
To study the drug release profile of synthesized PNIPAAm-MAA-VP-coated MNPs, drug-loaded nanoparticles dispersed in PBS as described earlier were placed inside dialysis bags with a molecular weight cutoff (MWCO) of 2000 Da. Samples was incubated at 37˚C. At designated time intervals, 2 mL of dialysate was removed from sample and stored at –20˚C for later analysis. Dialysate volume was reconstituted by adding 2 mL of fresh PBS to each sample. After the experiment the dialysate samples were analyzed using a UV-Vis spectrofluorometer (Tecan) to determine the amount of DOX released into the dialysate (λe 483nm DOX measurement).
2.7. Characterization of Magntic Poly (NIPAAm-MAA-VP)
2.7.1. Fourier Transforms Infrared Spectros Copy (FT-IR)
FT-IR spectra obtained by spectrophotometer (Shimadzu 8400, Kyoto Japan) for blank and drug loaded nanoparticles using KBr discs. The spectrum was taken from 4000 to 400 cm−1.
2.7.2. 1H-NMR Spectroscopy
The chemical structures of the copolymers were determined by 1H-NMR (Bruker spectra spin 400 MHz, Leipzig, Germany).
2.7.3. LCST Determination
The lower critical solution temperature (LCST) of all the terpolymers was measured by cloud point (turbidity) measurements. The inflection point of the turbidity curves was taken for the value of the LCST of the polymers. The optical transmittances of these solutions were measured at 500 nm wavelength using UV-vis spectrometer (UV-160, Shimadzu) with increasing solution temperature (20-408C, 18C interval). At each temperature, the samples were stabilized for 5 min before measurements. Values for the LCST of polymeric solutions were determined at temperature with a half of the optical transmittance between below and above transitions.
2.7.4. Determination of Particle Size and Morphology of the Drug-Loaded Nanoparticle
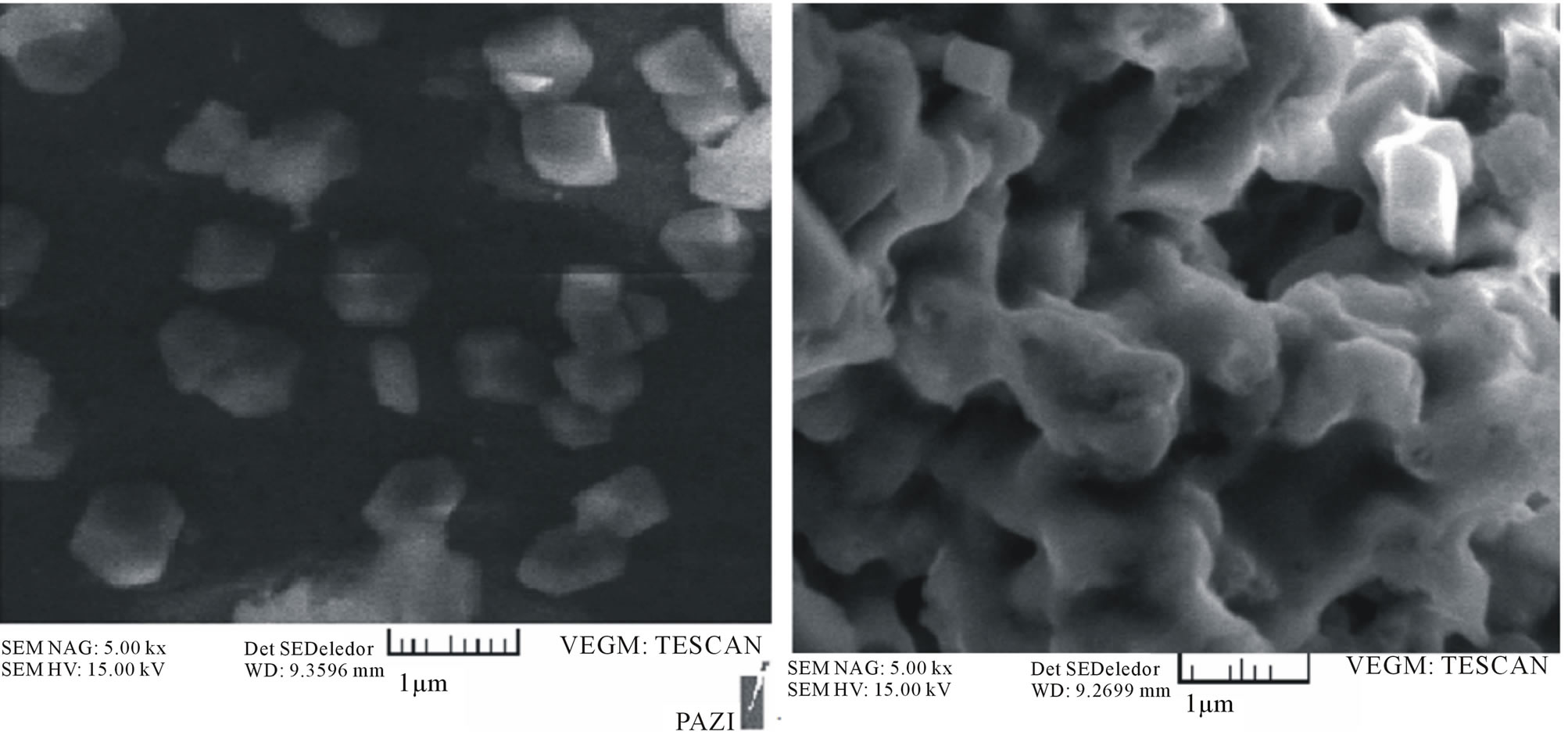
Measurement of the average diameter of nanoparticles was performed in de-ionized water by the dynamic light scattering technology (Nanotrac. 150, Microtrac Inc.). The size measurement was performed at room temperature (25˚C) and morphology of the polymeric nanoparticles was observed using a scanning electron microscopy (SEM, Leo Electron Microscopy Ltd, Cambridge ,UK). For the scanning electron microscopy (SEM), the lyophilized nanoparticles were placed on a double stick tape over aluminum stubs to get a uniform layer of particles. Samples were then gold coated using a sputter gold coater. Gold coated particle samples were cooled over liquid nitrogen prior to SEM observations to avoid their melting under high magnification due to the electron beam exposure. Prior to examination, samples were prepared on aluminum stubs and coated with gold under argon atmosphere by means of a sputter coater.
3. Result and Discussion
3.1. Particle Size, Size Distribution, and Morphology
The average size of the synthesized NIPA-MAA-Vp nanoparticles was analyzed by using dynamic light scattering technology. This type of relationship between surfactants and the nanoparticle size is consistent with previous observations.
However, the relationship was found to be nonlinear at SDS concentrations higher than 0.298 mM. Since we selected 100-nm nanoparticles for later studies, several characterization techniques were performed for this particular size only. The nanoparticle size distribution of 100 nm particles is shown in Figure 2. The size of NIPA-MAA-VP nanoparticles was also analyzed using SEM.
SEM revealed that the preparation procedure gave spherical nanoparticles. The size noted by SEM was within the range of size measured by Nanotrac.
3.2. Characterization of Copolymers
The chemical composition of the synthesized nanoparticle was analyzed using both FTIR and NMR. As shown in Figure 4 for FTIR, the stretching vibration appearing in the range of 2900 - 3000 cm−1 corresponds to C–H bands. The IR peaks at 3400 cm−1and 3450 cm−1 corresponds to the stretching vibration of the primary amine group in the NIPA-MAA-VP. Strong peaks in the range of 800 - 1000 cm−1 correspond to the stretching mode of vinyl double bonds disappeared in the spectrum of polymer indicating that polymerization has taken place. Furthermore, the carbonylgroup of NIPA and MAA is observed at 1680 cm−1.
In order to analyze the chemical composition of the NIPA-MAA-VP in more detail, proton (1H NMR) used. In 1H NMR (Figure 5), several characteristic peaks from NIPAAm, AAm and VP were overlapped. But the signals pertaining to NIPAAm are found in 1.3 ppm related to (CH3)2CH and in 4 ppm related to N-CH-(CH3)2 and the signals pertaining to VP are found in, 3.3 ppm corresponded to (N-CH2).
3.3. LCST Determination
To determine the temperature at which the phase transition occurs in the nanoparticles, UV-Vis spectrophotometer was used. As shown in Figure 6, the LCST of was 42˚C - 43˚C. The rate at which the transition occurs slowly changes around 39˚C, and then the intensity sharply decreases at 42˚C.
3.4. Drug-Loading Efficiency and Release Kinetics
The loading efficiency of DOX-loaded NIPAAm-MAA -VP coated MNPs was determined according to the formula illustrated earlier in the Methods section. The results indicated that 68% of the incubated DOX was loaded into the NIPAAm-MAA-VP coated MNPs, The release behavior of the nanoparticles was studied for 9 days in PBS (0.1 M, pH 7.4) at 37˚C,
3.5. Study of Loading and Drug Release Profile from Hydrogels
In table 2 physicochemical properties of lattice Hydrogels containing the doxorubicin hydrochloride drug are summarized.
The table shows that the lattice structure of the drug can lead to more incarceration. Diagram of drug release in pH = 4/7 is given in figure 7. Release curve shows that a high percentage of the drug after about 200 hours released with approximately linear process. Also initial burst of drug release is not observed.

Figure 2. Particle analyzer diagram of P(NIPAAm-MAA-VP) coated MNP.
 (a) (b)
(a) (b)
Figure 3. SEM of magnetic P(NIPAAm-MAA-VP) nanoparticles.

Figure 4. FT-IR spectrum of P(NIPAAm-MAA-VP).
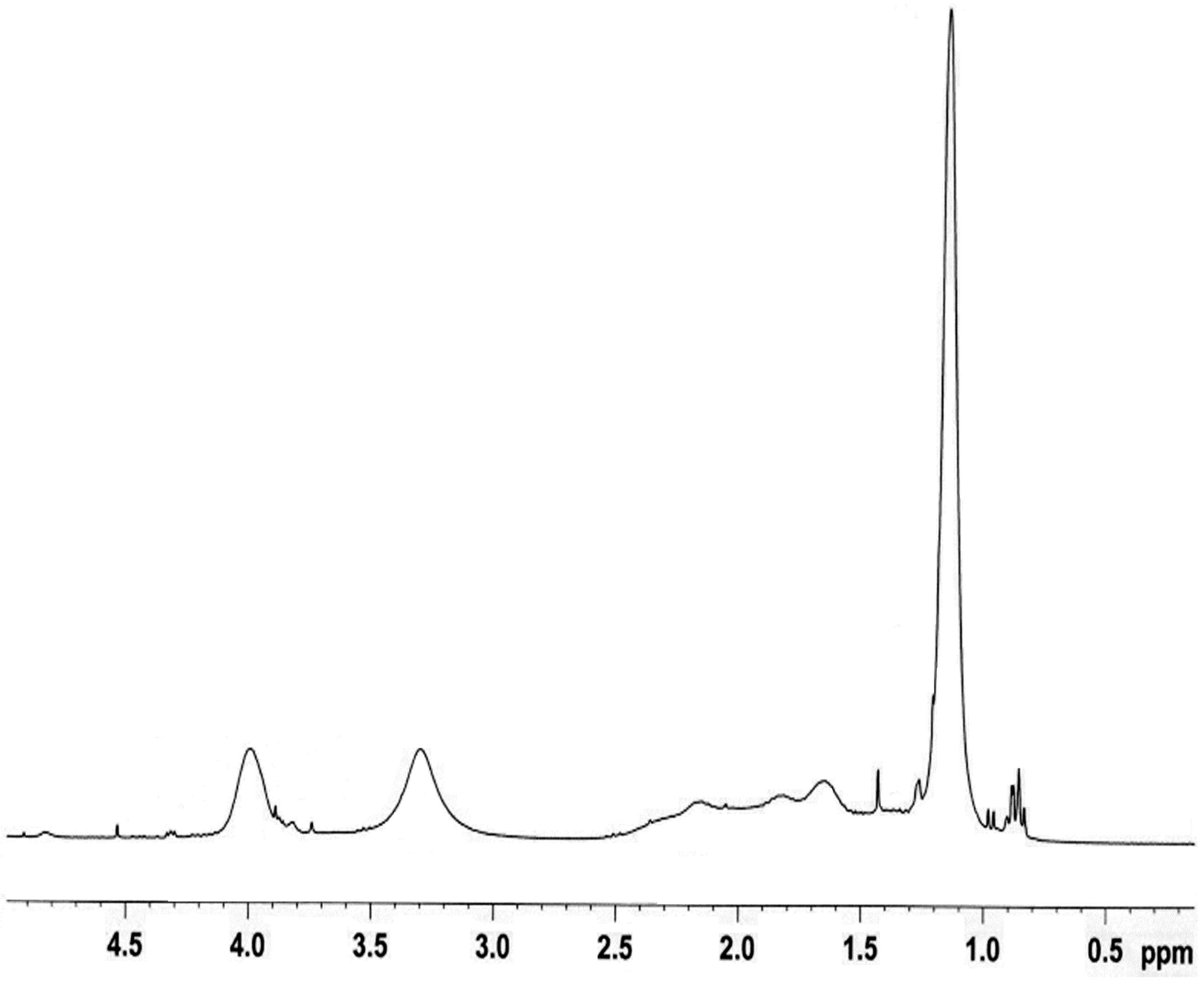
Figure 5. 1H-NMR spectrum of P(NIPAAm-MAA-VP).

Figure 6. LCST of nanoparticles measured by using UV-Vis spectrophotometer.

Figure 7. The release profile of doxorubicin hydrochloride copolymer lattic P(NIPAAm-MAA-VP).

Table 2. Physical characteristics of synthesized polymers.
4. Conclusion
In this work, we have characterized in vitro behavior of PNIPAAm-MAA-VP-coated MNPs for targeted and controlled drug delivery applications. These nanoparticles are to our knowledge unique, in that they consist of a new temperature-sensitive polymer shell that has the LCST above body temperature and contains functional groups on their surface for bioconjugation. The polymeric shell consists of a copolymer of NIPA, MAA, and VP. The PNIPAAm-MAA-VP is polymerized onto the surface of the MNPs and a free-radical polymerization. The size and morphology of the synthesized nanoparticles were analyzed by particle size analyzer. In addition, in vitro behaviors such as drug-loading efficiency and drug release profile were assessed. The results are discussed in detail below. SEM was carried out to study the morphology and the core shell structure of the nanoparticles [20-23].
The drug release study indicates that the PNIPAAmAAm-VP is a temperature-sensitive polymer, whereby at its LCST, the nanoparticles go through the phase change to collapse and release more drugs. After 200 hours, 92% of the encapsulated DOX was released at 37˚C [24-31].
5. Acknowledgements
The authors thank Department of Medical Nanotechnology, Faculty of Advanced Medical Science of Tabriz University for all supports provided.
NOTES
Authors’ contributions: SD conceived of the study and participated in its design and coordination. AA participated in the sequence alignment and drafted the manuscript. All authors read and approved the final manuscript.
#Corresponding author.