Investigation of Inlet Gas Streams Effect on the Modified Claus Reaction Furnace ()
1. Introduction
Hydrogen sulfide (H2S) is produced from sulfur compounds in fossil fuels such as natural gas or oil. Sour gases (H2S and CO2), are removed from the natural gas or refinery gas by means of one of the gas treating processes. Due to global environmental rules, refineries have to recover sulfur from nature. H2S containing acid gas stream is flared, incinerated, or fed to a sulfur recovery unit. The Claus process is commonly used to reduce the emission of sulfur compounds into the atmosphere. Recently recovery of sulfur is done by means of the modified Claus tail gas clean-up processes. In these processes, H2S over a catalyst converts to elemental sulfur where the reaction takes place in a high temperature furnace. The recovery process is the reaction between H2S and air to form sufur and water. Following reaction is the main reaction in the recovery process:
 (1)
(1)
In the original Claus process, control of this reaction was difficult and sulfur recovery efficiencies were low. In order to overcome these difficulties and also increase the efficiency of the process, several modifications of the Claus process have been developed. In modified process, free flame total oxidation of 1/3 of the H2S to SO2 followed by a reaction over the catalyst of SO2 with the remaining 2/3 H2S. According to Mohamed Sassi and Ashwani K. Gupta modified Claus process for a Sulfur Recovery Plant consists of several stages [1]:
1) Combustion (In the Reactor Furnace)
 (2)
(2)
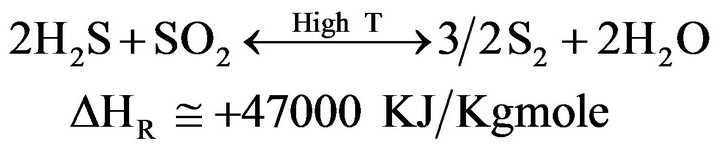 (3)
(3)
2) Redox (Catalytic Converter)
These are simplified reactions which actually take place in a Claus unit. There are various species of gaseous sulfur S2, S3, S4, S5, S6, S7, and S8. Equilibrium concentrations of these sulfur compounds are not known in the
 (4)
(4)
 (5)
(5)
entire of process. Additionally, gas stream contains water saturated with 15 - 80 mol% H2S, 0.5 - 1.5 mol% hydrocarbons, and CO2 which can result in carbonyl sulfide (COS) carbon disulfide (CS2), carbon monoxide (CO), and hydrogen [2].
Most Claus plants operate in the multistep process “straight-through” mode as shown in Figure 1. The combustion is carried out in a reducing atmosphere with only enough air 1) to oxidize one-third of the H2S to SO2, 2) to burn hydrocarbons and mercaptans, and 3) for many refinery Claus units, to oxidize ammonia and cyanides. The process includes the following operations:
• Combustion: burn hydrocarbons and other combustibles and oxidize one-third of the H2S to provide necessary SO2 to react with remainder H2S for producing S2 in the furnace.
• Waste Heat Recovery: Cool combustion products.
• Sulfur Condensing: Cool outlet streams from waste heat recovery unit and from catalytic converters.
• Reheating: Reheat process stream, after sulfur condensation and separation, to a temperature high enough to remain sufficiently above the sulfur dew point.
In order to investigate different aspects of the modified Claus process, a number of studies have been performed on main burner and sulfur recovery in this process. Monnery et al. modeled the modified Claus process [3]. Kelly Anne Hawboldt has studied mathematical modeling of reactions in the process [4]. Recently, S. Asadi et al. used TSWEET simulator to optimize the recovery of sulfur [5].
At first approach, we have used a mathematical model for the key reactions that take place in the reactor furnace. In the second approach, we have simulated the process with a commercial simulator. Finally using the model and simulation, we have compared obtained results and proposed some improvements on the base case.
2. Kinetic Studies
Claus process has been investigated via different aspects, experimental and theoretical perspectives. Paskal et al. gives a summary of the main reactions thought to occur within the Claus furnace [6,7]. Clark et al. discussed the mechanisms behind the formation of key sulfur containing species found within the furnace, and in a subsequent study outline primary reaction pathways for the principal components in the furnace [8,9]. While there have been numerous attempts to model the Claus process based on simplified kinetic expressions, the complexity of the chemistry and the number of involved reactions has precluded the accurate prediction of outlet compositions. As it mentioned before, gas stream contains different compounds such as H2S, CO2 and hydrocarbons. Most important compound is H2S and several groups have studied it’s decomposition under different condition. As result, it suggested that there are numerous reactions on the catalytic decomposition of H2S in the clause process. According to the studies, gaseous H2S exists in chemical equilibrium with elemental hydrogen and sulfur by the following equation:

Figure1. . Three-stage straight-through sulfur plant.
 (6)
(6)
Oxidation includes two staged reaction, first oxidation of H2S and followed by reaction between H2S and SO2 that limiting stage of the Claus reaction is the second part [10].
 (7)
(7)
 (8)
(8)
During the reaction in the furnace and according to the existence of ammonia in the gas stream, oxidation of NH3 will take place. Recently, Clark has mentioned that under 1100˚C ammonia oxidation is negligible. Additionally, he noted in competitive oxidation, first of all H2S, and then methane, finally NH3 react. On the other hand, Goar et al. found rate of hydrocarbon combustion is more than ammonia and NH3 is more than H2S [11]. Formation of COS and CS2 in the Claus reaction furnace are also very important in the modeling. Field studies have revealed that concentrations of COS and CS2 at the exit of the reaction furnace/ waste heat boiler typically lie between 100 ppm and 2 mol% [12]. However, these seemingly small concentrations in the furnace product stream can represent nearly half the sulfur emissions from a tail gas clean-up unit [13]. It is possible to hydrolyze COS and CS2 back into H2S in the Claus catalytic converters according to the following stoichiometry:
 (9)
(9)
 (10)
(10)
As it mentioned, there many reactions which may take place in the furnace according to the conditions such as temperature and pressure. Full list of reactions that occur in the furnace is not obvious and for the known reactions, reaction rate expressions are not available. In current work we assumed that gas stream consists of CH4, CO2, H2S, H2O, O2, N2, CO, CS2, COS, S2, SO2, H2. Regarding gas stream composition, important reactions which take place in furnace and we use in the modeling are listed below.
 (11)
(11)
 (12)
(12)
 (13)
(13)
 (14)
(14)
 (15)
(15)
 (16)
(16)
 (17)
(17)
3. Mathematical Model
The basic structure of the model consist of the equations of mole and energy conservative rule the furnace, which are related to each other and are function of molar conversion of H2S in equilibrium reaction and temperature. In order to model the reactor, a steady-state simulation has been used for mole and energy balance.
Sames and Paskal presented empirical correlations to predict the fraction of CO, H2, COS, CS2 and sulfur (as S) in the effluent of the Claus furnace. The correlations are obtained from more than 300 tests on 100 different sulfur trains; with different flow configurations processing acid gas feed streams [12]. These empirical correlations are presented in Appendix. We use these equations to model the furnace and mole balance. In this work, furnace pressure is 130 kPa (absolute) and pressure drop (ΔP) is 10 kPa. Using empirical equations and applying in the mole balance for the compounds, we get the mole balance equations, for each compound.
For gas components in the outlet gas stream:
H2S:
 (18)
(18)
H2O: (19)
(19)
CO2:  (20)
(20)
CO:  (21)
(21)
SO2:  (22)
(22)
CH4: 0 (23)
O2:  (24)
(24)
N2:  (25)
(25)
S2:  (26)
(26)
H2:  (27)
(27)
CS2:  (28)
(28)
COS:  (29)
(29)
Total moles:
 (30)
(30)
X: Flow rate of H2S conversion at the equilibrium Claus reaction At equilibrium:
 (31)
(31)
By interpolation in the Kp curve vs. furnace temperature (GPSA), we calculate:
 (32)
(32)
Replacing molar flows in equation (18) through 30 in equation (31) resulted into a new equation, function of temperature, in the following form (see below):
where the A, B, C, D are function of feed composition and would be calculated numerically.
In order to calculate the reactions enthalpy, we used equation 34 and data provided in table 1. Table 1 represents standard enthalpy of formation and heat capacity parameters for each component.
 (34)
(34)
∆Hrxn is standard enthalpy of reactions in 25˚C, frxn(i) is molar flow rate of limiting compound in each reaction, Conversioni is conversion value of limiting compound in each reaction.
Replacing all mentioned equations resulted into a new equation (35) in which conversion of H2S is function of temperature of reaction furnace (table 2).
 (35)
(35)
A0, A4, A5, B0, B4, B5, C4, C5, D4, D5 are constant parameters that would calculated numerically according to the inlet gas stream condition.
In order to establish a reference point, calculations are carried out for a “base case” and the operating conditions used are given in Table 3. Our base case is Shahid Hasheminejad Gas Refinery. Shahid Hasheminejad (Khangiran) gas refinery is in Sarakhs, Khorasan province.
Operating conditions is composition of inlet gas stream to the Claus process in the refinery. Next step, application of the feed gas condition in the equations resulted into the parameters of mole and energy balance equations. Calculated parameters are presented in table 4.
4. Process Simulation
In order to compare the implications of the reaction fur-

Table1. . Compound heat capacity parameters and H2980 kJ/kg mole.

Table 2. limiting specie and calculated ΔHrxn [4].

Table 3. Refinery claus process inlet gas condition.
 (33)
(33)

Table 4. Calculated parameters for case study.
nace model on design, overall sulfur recovery and emissions, this modified Claus plant was simulated using SULSIM®.
SULSIM® is program for Sulfur Plant Simulation and represents simulation package for SRU and TGTU design. This software has widely accepted thermodynamic data and propriety thermodynamic properties for all of the gas components and sulfur species found in sulfur recovery processes. Figure 2 shows the flow diagram of the simulated Claus unit of Shahid Hasheminejad Gas Refinery.
5. Results and Discussion
As described previously, we implemented real data from gas refinery in the model. In this section to verify the model, we compare the output result from reaction furnace, model values and simulation results. Table 5 listed three set of results.
It is obvious that furnace temperature obtained using model (1098 K) is lower than actual temperature of reaction furnace (1113 K), as the 15˚C temperature difference is negligible and it results into 1.35% error. Simulated results shows that predicted temperature using SULSIM® software is 1121 K and higher than Claus furnace temperature. Error occurred using software is lower (0.72%). In this case simulation is more reliable. Additionally, sulfur conversion obtained from model results (56.635%) is in good agreement with conversion of sulfur in Claus plant (54%) in gas refinery. On the other hand, results from simulation indicate that sulfur conversion is 60.28%. According to the fact that obtained conversions from model are attained in lower temperature in comparing with the plant temperature while sulfur product is higher, we can conclude that model is more efficient in sulfur conversion. It is obvious that presented model in these conditions, is effective even more than simulated process. As it is presented in the table 5, in the furnace effluent there is sulfur vapor. It’s due to the high temperature in the furnace; this high temperature converts sulfur to S2 vapor. According to table 5, inlet air ratio to acid gas feed in Claus plant is 0.86 and in our model this ratio is 0.83. As we used this obtained ratio in our simulation, simulation and modeling resulted in the same ratio. Therefore modeling and simulation errors are low and about 3.5%. Since sulfur production is high in comparing with actual plant; air consumption is low, CO2 concentration difference is about 0.05 (mole %), it means error is 0.34%; we can conclude that in all cases, simulated and modeling are more efficient.
Predicted N2 concentration using equilibrium model is 36.067 (mole %) whereas N2 content in the plant outlet gas stream is 39 (mole %). Since In empirical equations of model, it is assumed air consumption is low, results are logic and difference between real state and model results is acceptable. Since air consumption is low, its acid gas capacity is more than actual plant and can predict better results. Simulation has the same manner in the prediction of N2 concentration. H2O concentration in the outlet stream from model is less than plant outlet water content. The model performance was not good in water case and error value is about 7.8%. While H2O content in simulation is closer to the actual data and lower error has occurred. Simulation performance is better in this case.
O2 component in Claus plant damages the equipments (catalyst exchanger) and must be minimized, in Claus plants O2 content is zero. Predicted concentration is

Figure 2. SULSIM® simulation used for gas for Khangiran gas refinery (S.G.P.C).

Table 5. Comparison of plant value and results from model and simulation for S.G. P.C.
0.014% and error value is acceptable. The model predicted CH4 concentration would drop to zero in the outlet of furnace. Checking the actual outlet concentration it is obvious that there is no methane in outlet stream, therefore no error has occurred in predicting of methane concentration. Table 5 represents actual data for the concentration of CS2 and COS are greater than equilibrium model, data obtained from simulation. As you know, production of CS2 and COS in the Claus furnace reduces the efficiency of total plant and it is better to decrease these components. Therefore simulation and model outputs are more effective. According to Sames (1990), COS forms in WHB exchanger, therefore difference between predicted concentration and plant data verifies the formation reaction of COS [14]. This take place in W.H.B as following equation:
 (40)
(40)
Predicted S2 content in both methods is greater than plant data. There is a big difference between real and predicted values for S2; it is due to the formation of liquid sulfur in WHB. According to table 5 and comparison between results and plant data, and also neglecting the error in CO and H2 predicted concentrations, average error is about 3.5% and 5.36% for model and SULSIM® simulation; also AAD (Average Absolute Deviation) in comparing actual data with modeling and simulation results are 2.07% and 4.92%, respectively. We can conclude that our model is more efficient and applicable for other Claus plants with different inlet composition.
5.1. H2S Concentration Effects
Figure 3 shows reaction furnace predicted temperature vs. inlet H2S content using model and simulation. Both simulation and model have similar trend. According to the figure, model predicts that 1% increase in H2S content will result into 7.5˚C increase in furnace temperature. In order to combust hydrocarbons and aromatics, furnace temperature must be 1050˚C. According to the model, if inlet gas stream contains more than 26% H2S in current plant, temperature would increase to higher than 1050˚C (1323 K). Figures 4(a) and (b) illustrate the predictions of model and simulation for H2S and sulfur conversion in furnace vs. increase in H2S content in feed. As model predicts, for one percent of the mole fraction of H2S in feed stream, sulfur conversion increases by 0.54% in reaction furnace and S2 mole fraction in outlet gas stream increases 0.12%. Simulation has similar manner in this case. Obtained results both are matched. It should be noted that sulfur conversion in figure 4(b) shows model and simulation have similar trend. Therefore there is a negligible difference between the model and simulation prediction in 18% H2S in figure 4(a). It is due to errors occurred in the simulation.