1. Introduction
Obesity is a disorder involving excessive body fat that increases the risk of health problems [1]. In 2020, the World Health Organisation estimated that more than 1.9 billion adults were overweight; of these, 650 million were obese worldwide while over 340 million children and adolescents aged 5 - 19 were overweight or obese. An estimate of 39 million children under the age of 5 was overweight or obese [2]. Obesity is a risk factor for cardiovascular disease, diabetes mellitus, fatty liver disease and cancers [3].
Obesity is a multi-factorial disorder resulting from combination of several acquired and genetic factors. Acquired causes of obesity include sedentary lifestyle and over-nutrition. Genetic causes of obesity include dysmorphic syndromes, mutations in leptin, adiponectin and PPAR-γ genes as well as overexpressin of neuropeptide Y (NPY) [4].
The existing drugs for treating obesity have been effectively implemented in regulated weight-loss treatments. However, they pose severe adverse effects such as gastrointestinal, kidney and heart problems which outweigh their advantageous properties. Most recent studies on the treatment of obesity have been based on the potential role of different plant preparations that can exert a positive effect on the mechanisms involved in this pathology. The property is mainly due to various nutrients and phytochemicals found in plants [5].
Chrysophyllum albidum (white star apple) is a medicinal plant found in the tropical and sub-tropical parts of the world [6]. In folklore medicine, bark of Chrysophyllum albidum is used in treating yellow fever and malaria [7]. The leaf is used for providing relief from stomach ache and diarrhea [8]. Reports have shown that extracts of Chrysophyllum albidum have hepatoprotective, antiplasmodial, antibacterial hypoglycaemic and hypolipidaemic properties [9] [10] [11] [12]. Similarly, Irvingia gabonensis has several medicinal uses. The stem bark is ingested in order to treat hernia, yellow fever, and diarrhea [13]. Reports have shown that the stem bark and fruit juice have antidiabetic and antihyperlipidaemic effects in rats [14] [15].
There is paucity of scientific information on the anti-obesity potential of the leaves of these plants. Also, these leaves contain tannins, flavonoids, alkaloids and saponins [16]. These phytochemicals have been said to have antiobesity property [17].Therefore, this study sets out to compare the effect of methanol leaf extract of Chrysophyllum albidum and Irvingiagabonensis on obesity induced in Wistar rats using a high fat diet.
2. Materials and Methods
2.1. Chemicals and Reagents
Alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin, total protein, albumin, triacylglycerol, HDL cholesterol, urea, creatinine and total cholesterol kits were products of Randox Laboratories, UK while orlistat® (Ecoslim) drug was product of Micro Laboratory Ltd, India.
2.2. Animals and Housing
Male albino rats (Wistar strain, 70 - 90 g), bred in the Department of Biochemistry, Faculty of Life Sciences, University of Benin, Benin City, Edo State, were utilized for this research. All animals were allowed to acclimatize for two weeks before being used for the study. The experiment was carried out in line with internationally recommended principles for laboratory animals’ use and care according to Canadian Council on Animal Care Guidelines and Protocol Review [18].
2.3. Collection of Plant Materials
The leaves of Chrysophyllumalbidum and Irvingiagabonensis used in this study were collected from a private farm at Ewu, Esan Central Government Area of Edo State, Nigeria. The fresh leaves were identified by Prof. M.E. Osawaru and further authenticated by Professor MacDonald Idu both of the Department of Plant Biology and Biotechnology of the University of Benin, Benin City, Nigeria. Herbarium specimen (voucher numbers UBHc362 and UBHi 153) of both plants Chrysophyllumalbidum and Irvingiagabonensis, respectively) were deposited at the Herbarium of the same department.
2.4. Preparation of Plant Extracts
The leaves were rinsed properly with water and air-dried away from direct sunlight. The dried leaves were pulverized, soaked in methanol for 72 hours and filtered with a double layered muslin cloth. Each filtrate was then dried using a rotary evaporator at 40˚C and stored in air-tight sterile bottles for subsequent analyses [19].
2.5. Acute Toxicity Study
Acute toxicity test was performed on the extracts to determine the range of lethal doses (LD50), according to method of OECD [20]. A total of eleven groups with three male rats (110 - 130 g) were utilized. Ten groups were orally administered varying doses (100, 250, 500, 1000 and 2000) mg/kg of Chrysophyllumalbidum or Irvingiagabonensis extract dissolved in the vehicle (carboxylmethyl cellulose) while the eleventh group (control) received equivalent quantity of the vehicle only. After administering the extracts, the general behavior of the animals was observed at 0 and 30 min and 1, 2, 4 and 24 hours for physical signs of toxicity and mortality. The rats were checked closely for 14 days for signs of any physical changes such as excitation, fatigue, diarrhea, itching, curved tail, shivering, falling of hair and mortality. All the animals had free access to food and water throughout the period of the study [21].
2.6. Induction of Obesity
In this study, high fat diet (HFD) was used to induce obesity in the experimental rats (Table 1). Seventy male rats were distributed into seven groups of ten animals each as follows:
Group 1: Served as the normal control and were fed with normal fat diet for 8 weeks.
Groups 2, 3, 4, 5, 6, 7: Animals were fed with high fat diet for 8 weeks in order to induce obesity. Body weight of all animals was measured weekly. At the end of eight weeks, lipid profile, mean body weight, adiposity, body mass index and Lee’s index were measured in order to evaluate induction of obesity in HFD groups.
2.7. Treatment of Obese Animals
After inducing obesity and hyperlipidaemia, treatment of the rats in the various groups was carried out as follows for additional 4 weeks (weeks 9 - 12):
Group 1: Represented the normal control. The animals were fed on the normal fat diet (NFD) and were administered orally with the vehicle (carboxy methyl cellulose 0.5% solution).
Group 2: Served as the negative control. The high fat diet (HFD) fed rats were treated with only the vehicle.
Group 3: The HFD fed rats were administered orlistat (anti-obesity reference drug 20 mg/kg, p.o).
![]()
Table 1. Composition normal fat diet (NFD) and high fat diet (HFD) [22].
Group 4: The HFD fed rats were treated with methanol leaf extract of C. albidum (250 mg/kg, p.o).
Group 5: The HFD fed rats were treated with methanol leaf extract of C. albidum (500 mg/kg, p.o).
Group 6: The HFD fed rats were treated with methanol leaf extract of I. gabonensis (250 mg/kg, p.o).
Group 7: The HFD fed rats were treated with methanol leaf extract of I. gabonensis (500 mg/kg, p.o).
Water and food were given to the animals ad libitum throughout the period of study; their body weight (g) was measured weekly while food intake was recorded daily.
2.8. Collection of Blood Samples
At the end of the experiment, the animals were fasted overnight and sacrificed by cervical dislocation, under chloroform anaesthesia. Blood was taken via cardiac puncture into sterile tubes and allowed to stand for 30 minutes at 20˚C - 25˚C. The clear serum was separated at 2500 g for 15 minutes using a centrifuge. The serum samples were stored in a freezer and thereafter used for biochemical analyses.
2.9. Histological Analyses
Liver and kidney were harvested from the animals and used for histological testing. The organs were taken and dipped in 10% Phosphate buffered formalin solution. The organs were cut into bits, rinsed and dehydrated in ascending grades of alcohol. Dehydrated specimens were cleared in xylol, embedded in paraffin, sectioned at 4 - 6 microns thickness and stained with Heamtoxylin and Eosin for histopathological analyses [23].
2.10. Biochemical Analyses
The following procedures were used to perform the analyses:
· Alanine aminotransferase (ALT) [24].
· Aspartate aminotransferase (AST) [24].
· Alkaline phosphatase (ALP) [25].
· Bilirubin [26].
· Total protein [27].
· Albumin [28].
· Total cholesterol [29].
· Triacylglycerol [30].
· High density lipoprotein (HDL) Cholesterol [30].
· Urea [31].
· Creatinine [32].
· Creatine kinase [33].
· Lactate dehydrogenase [34].
The low density lipoprotein (LDL) cholesterol and Very low density lipoprotein (VLDL) cholesterol concentration were estimated using the formulae by Friedewald [35] as shown below:
· LDL cholesterol = Total Cholesterol − (HDL cholesterol + Triacylglycerols/5).
· VLDL cholesterol = Triglycerides/5.
2.11. Estimation of Anthropometric Parameters and Adiposity
The body length (nose-to-anus length) and weight were measured in anaesthetized rats and values obtained were used to calculate the body mass index (BMI) and Lee’s index [36].
Body mass index (BMI) = Body weight (g)/Body length2 (cm2).
Lee’s index = Cube root of body weight (g)/Nose-to-anus length (cm).
Furthermore, epididymal fat and retroperitoneal fat were dissected and weighed. Values obtained were used to calculate the adiposity index of the animals [37].
2.12. Statistical Analysis
Data were statistically analyzed using the Statitical Package for Social Sciences (SPSS) version 17.0 for windows. One way analysis of variance (ANOVA) was used to compare standard error of mean. Values were considered significant at p < 0.05. Inter-group comparisons were performed by using the Tukey’s post hoc test.
3. Results
3.1. Results for Induction of Obesity
Table 2 shows the serum lipid profile of experimental rats evaluated at week 8 of feeding with high fat diet to confirm induction of hyperlipidaemia and obesity. The total cholesterol, triacylglycerols, LDL cholesterol and VLDL cholesterol in groups of rats fed with the high fat diet (HFD) were significantly (p < 0.05) higher than those of rats fed with the normal fat diet (normal control). These changes indicated the induction of hyperlipidaemia in the animals. In contrast, the HDL cholesterol levels were significantly (p < 0.05) lower in all the high fat diet exposed rats in contrast to the normal control.
The body mass index (BMI) and the Lee’s index of rats in the HFD fed groups were significantly (p < 0.05) higher than that of the normal control (Table 3). The Lee’s indices of the HFD fed groups were greater than 0.3 suggesting induction of obesity while the normal control showed Lee’s index value of less than 0.3. Table 4 depicts the adiposity of rats after induction of obesity. The weights (g) of the epididymal fat, retroperitoneal fat and adiposity index of rats in the
![]()
Table 2. Lipid profile (mg/dL) of rats in various groups after eight weeks of induction of obesity.
Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters in same column indicate significant difference between means at p < 0.05. After induction of hyperlipidaemia and obesity, the experimental rats were assigned into various treatment groups for four weeks. NFD = Normal fat diet; HFD =High fat diet (negative control).
![]()
Table 3. Body mass index and lee’s index of rats after eight weeks of induction of obesity.
Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters in same column indicate significant difference between means at p < 0.05.
HFD fed groups were significantly (p < 0.05) higher than the normal control.
3.2. Body Weight
Figure 1 shows the weekly body weight of rats for the 12 weeks experimental period of the study. Results showed that between weeks 5 to 8, the mean body weight of rats in the high fat diet (HFD) groups were significantly (p < 0.05) increased than the normal fat diet (NFD) fed control group, suggesting induction
![]()
Table 4. Epididymal fat, retroperitoneal fat and adiposity index of rats after eight weeks of induction of obesity.
Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters in same column indicate significant difference between means at p < 0.05.
![]()
Figure 1. Effect of C. albidum and I. gabonensis on weekly body weight of rats Values are expressed as mean ± SEM. # = significantly different (p < 0.05) from normal fat diet control during induction; * = significantly different (p < 0.05) from high fat diet control during treatment.
of obesity in the HFD groups. During the period of treatment (week 9 to 12) with the extracts or reference drug (orlistat®), the body weights of the rats were significantly (p < 0.05) reduced in comparison to the high fat diet control.
3.3. Lipid Profile, Atherogenic Index and Coronary Risk Index
Table 5 depicts the effect of C. albidum and I. gabonensis extracts on lipid profile
![]()
Table 5. Effect of C. albidum and I. gabonensis extracts on lipid profile (mg/dl) of obese rats.
Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters in same column indicate significant difference between means at p < 0.05. Normal Control = Normal Fat Diet; HFD = High Fat Diet.
of normal and obese rats. Results showed significantly (p < 0.05) higher total cholesterol, triglycerides, LDL cholesterol and VLDL cholesterol levels in the high fat diet (HFD) fed untreated rats when compared to the normal control. However, treatment of the obese rats with the extracts or orlistat® showed reversal in the levels of these parameters and were comparable to the normal control. The HDL cholesterol concentration was significantly (p < 0.05) reduced in the HFD group in comparison with the other groups.
Atherogenic index (Figure 2) and coronary risk index (Figure 3) were significantly (p < 0.05) increased in the HFD fed rats while the extracts treated as well as the orlistat® treated groups had normal values of these parameters comparable with the normal control values.
3.4. Body Mass Index and Lee’s Index
Administration of high fat diet (HFD) to rats resulted in significant (p < 0.05) increase in BMI and Lee’s index in comparison with the normal fat diet (NFD) fed group. Again, after treatment with the extracts, the BMI and Lee’s index levels of the rats returned to normal or close to normal levels (Table 6).
3.5. Adiposity
Treatment of the obese rats with either the C. albidum and I. gabonensis extracts
![]()
Figure 2. Effect of methanol extracts of C. albidum and I. gabonensis on Atherogenic index of obese rats. Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters on bars indicate significant difference between means at p < 0.05.
![]()
Figure 3. Effect of methanol extracts of C. albidum and I. gabonensis leaf on Coronary Risk Index (CRI) of obese rats. Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters on bars indicate significant difference between means at p < 0.05.
or the reference drug, orlistat resulted in significant (p < 0.05) reduction in weights (g) of the epididymal fat, retroperitoneal fat and adiposity index of rats when compared with the untreated HFD fed rats (Table 7).
3.6. Food Consumption
The induction of obesity in the groups of rats (with the exception of the normal fat diet fed rats) by using high fat diet for 8 weeks and their subsequent treatment with the extracts or reference anti-obesity drug (orlistat®) for another 4
![]()
Table 6. Effect of leaf extracts of C. albidum and I. gabonensis on body mass index and Lee’s index of obese rats.
Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters in same column indicate significant difference between means at p < 0.05. Normal Control = Normal Fat Diet; HFD = High Fat Diet.
![]()
Table 7. Effect of C. albidum and I. gabonensis Extracts on epididymal fat, retroperitoneal fat and adiposity index of obese rats.
Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters in same column indicate significant difference between means at p < 0.05. Normal Control = Normal Fat Diet; HFD = High Fat Diet.
weeks had no observable effect (p > 0.05) on their daily food intake when compared with the normal control (Figure 4).
3.7. Liver, Kidney and Cardiac Function Tests
The effect of C. albidum and I. gabonensis extracts on some liver function enzymes including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP) in normal and in obese rats is presented in Table 8. Results showed significant (p < 0.05) reduction in the activities of
![]()
Figure 4. Effect of C. albidum and I. gabonensis on food intake of rats. Data are expressed as mean ± SEM (n = 6 per group).
![]()
Table 8. Effect of C. albidum and I. gabonensis extracts on liver function enzymes of obese rats.
Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters in same column indicate significant difference between means at p < 0.05. Normal control = Normal fat diet; HFD = High fat diet.
AST, ALT and ALP of obese rats that were treated with C. albidum and I. gabonensis extracts and also those treated with orlistat® when compared with the untreated high fat diet (HFD) fed rats. Except for the ALP activity of the C. albidum (250 mg/kg) treated group which showed significant (p < 0.05) increase in the activity of this enzyme.
The effect of C. albidum and I. gabonensis extracts on other liver function parameters (bilirubin, albumin and total protein) are shown in Table 9. Total and conjugated bilirubin levels of the extracts and the orlistat® treated obese rats were markedly (p < 0.05) low in contrast to the untreated HFD fed counterpart. There were no noticeable changes (p > 0.05) in total protein and albumin concentrations in all the groups studied. The urea and creatinine levels were considerably (p < 0.05) increased in the untreated HFD fed rats in comparison with
![]()
Table 9. Effect of C. albidum and I. gabonensis extracts on serum bilirubin, albumin and total protein concentrations of obese rats.
Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters in same column indicate significant difference between means at p < 0.05. Normal control = Normal fat diet; HFD = High fat diet.
the normal control (Figure 5). However, treatment of the obese rats with extracts of C. albidum, I. gabonensis or orlistat® resulted in normal levels of these parameters.
Figure 6 depicts the effect of C. albidum and I. gabonensis extracts on serum creatine kinase and lactate dehydrogenase activities of obese rats. Results showed significant (p < 0.05) decrease in activities of creatine kinase and lactate dehydrogenase in the obese rats that were treated with either the C. albidum, I. gabonensis extracts or the anti-obesity reference drug, orlistat® when compared with the untreated HFD fed rats.
3.8. Histology
Histological section of the hepatocytes of rats fed with normal fat diet (NFD control) showed normal architecture. The hepatocytes were radially distributed around the central vein (Plate 1) as against the HFD control which showed hepatocytes having lymphoid cells aggregation and fat vacuoles (Plate 2). The hepatocytes section of rats in the extracts and the orlistat® treated groups revealed normal hepatocytes architecture similar to that of the normal control (Plates 3-7). The kidney section was normal for the rats fed on the normal fat diet (Plate 8) while that of the HFD control showed aggregation of the lymphoid cells (Plate 9). Similar histology of the kidney was noticed for the rats treated with orlistat® and the extracts treated groups with the kidney having normal glomeruli and tubules as the normal control group (Plates 10-14).
![]()
Figure 5. Effect of C. albidum and I. gabonensis extracts on serum urea and creatinine concentrations of obese rats. Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters on bars indicate significant difference between means at p < 0.05.
![]()
Figure 6. Effect of C. albidum and I. gabonensis extracts on serum creatine kinase and lactate dehydrogenase activities of obese rats. Data are expressed as mean ± SEM (n = 6 per group). Different lowercase letters on bars indicate significant difference between means at p < 0.05.
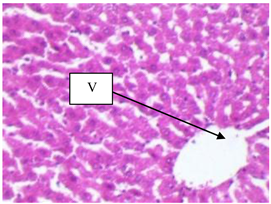
Plate 1. Photomicrograph section of the hepatocytes of rats fed on Normal Fat Diet (NFD control). Section of the hepatocytes shows normal architecture with central vein (V) [H & E ×400].
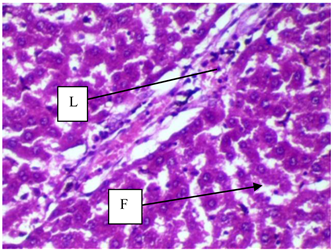
Plate 2. Photomicrograph section of the hepatocytes of rats in HFD control. Liver section of the rats fed on high fat diet reveals evidence of lymphocytic aggregation (L) and fat vacuoles in hepatocytes (F) [H & E ×400].
Plate 3. Photomicrograph section of the hepatocytes of rats treated with orlistat-20 mg/kg (H & E ×100).
![]()
Plate 4. Photomicrograph section of the hepatocytes of rats treated with C. albidum-250 mg/kg (H & E ×100).
![]()
Plate 5. Photomicrograph section of the hepatocytes of rats treated with C. albidum-500 mg/kg (H & E ×100).
![]()
Plate 6. Photomicrograph section of the hepatocytes of rats treated with I. gabonensis-250 mg/kg (H & E ×100).
![]()
Plate 7. Photomicrograph section of the hepatocytes of rats treated with I. gabonensis-500 mg/kg (H & E ×100).
![]()
Plate 8. Photomicrograph section of the kidney of rats in NFD control. Section of the kidney shows normal renal structure with Glomerulus (G) and Tubule (T) [H & E ×100].
![]()
Plate 9. Photomicrograph section of the Kidney of rats in HFD control. Kidney section of rats fed on high fat diet shows aggregation of lymphoid cells (L) [H & E ×100].
![]()
Plate 10. Photomicrograph section of the kidney of rats treated with orlistat-20 mg/kg (H & E ×100).
![]()
Plate 11. Photomicrograph section of the kidney of rats treated with C. albidum-250 mg/kg (H & E ×100).
![]()
Plate 12. Photomicrograph section of the kidney of rats treated with C. albidum-500 mg/kg (H & E ×100).
![]()
Plate 13. Photomicrograph section of the kidney of rats treated with I. gabonensis-250 mg/kg (H & E ×100).
![]()
Plate 14. Photomicrograph section of the kidney of rats treated with I. gabonensis-500 mg/kg (H & E ×100).
4. Discussion
This study compared the impact of leaf extracts of Chrysophyllumalbidum and Irvingiagabonensis on obesity induced by high fat diet in Wistar rats. Prior to the anti-obesity study, acute toxicity of the extracts was performed on Wistar rats. Results showed that both extracts were well tolerated by the rats even at the higher doses and no mortality was recorded in any group.
Studies have revealed that high fat diet promote hyperlipidaemia and obesity [38] [39] [40]. Thus, high fat diet may be employed in generating a rodent model for analyzing pathophysiology of hyperlipidaemia and obesity [41]. Similarly, in this study, obesity and hyperlipidaemia were induced by feeding rats with high fat diet for eight weeks. However, treatment of the obese rats with leaf extracts of Chrysophyllumalbidum andIrvingia gabonensis resulted in significant anti-hyperlipidaemic and anti-obesity effects that were comparable with the reference anti-obesity drug, orlistat®.
In this study, there was a significant (p < 0.05) reduction in total cholesterol, VLDL cholesterol, triacylglycerols, LDL cholesterol and increase in HDL cholesterol levels in the C. albidum and I. gabonensis treated obese rats in comparison with the HFD control. This supports previous report by [42]. In their study, treatment of obese rats with crude extract of medicinal plant led to an increased serum HDL cholesterol level and decreased levels of total cholesterol, LDL cholesterol and triacylglycerols. Atherogenic index (AI) and coronary risk index (CRI) are regarded as markers for various cardiovascular disorders. The higher their values, the higher the possibility of developing heart disease and vice versa [43]. In this study, it was observed that high fat diet intake resulted in increase in atherogenic index while treatment with the extracts significantly (p < 0.05) attenuated the observed high atherogenic index. Coronary risk index (CRI) was significantly reduced (p < 0.05) in the rats treated with methanol extracts of C. albidum, I. gabonensis in comparison with the negative control which showed high levels of CRI, thus, suggesting cardiovascular protective potential of the extracts. The ability of the extracts to restore normal lipid profile in the rats could be due to the presence of flavonoids in the extracts. Previous research has shown that leaves of Chrysophyllumalbidum andIrvingia gabonensis contain high levels of flavonoids [16]. Flavonoids have been said to prevent hyperlipidaemia by inhibiting lipogenesis and stimulating lipolysis [44].
Increases in weight and fat deposits are important signs for the progress of obesity. In this study, feeding the animals with the HFD led to an increase in adiposity, which caused an increase in size of the fat cell. Thus, there was an increase in body weight for rats fed with the HFD. This increase in body weight found in the rats that were fed with HFD may be because of the ingestion of food high in energy value, in the form of saturated fats (beef tallow) and its deposition in various fats pads of the body as compared to the normal fat diet (NFD) fed animals [45]. However, a significant decrease (p < 0.05) in body weight and fat mass (retroperitoneal as well as epididymal) was seen in the plant extracts and orlistat® treated rats. Comparatively, weight of retroperitoneal fat of rats treated with high dose (500 mg/kg) of Irvingiagabonensis leaf extract was significantly lower (p < 0.05) than that of rats treated with the leaf extract of Chrysophyllumalbidum. This suggests that Irvingiagabonensis leaf extract was more effective than Chrysophyllumalbidum leaf extract in suppressing adiposity.
A quick method for the determination of obesity in rats was described by Lee [46] and it was termed “Lee index”. Lee index and the fat mass have a correlation. It is employed as a fast and accurate way to measure obesity in rats. Lee’s index below 0.300 is considered normal [47]. Researchers have also suggested that another anthropometric parameter termed body mass index (BMI) can be employed to estimate body composition and obesity in rats [48]. Likewise, the present study revealed significantly (p < 0.05) higher BMI and Lee’s index of rats fed on the HFD when compared with the normal control. However, treatment of the obese rats with either the plant extracts or orlistat® resulted in significantly (p < 0.05) reduction in BMI and Lee’s index. Specifically, the Lee’s indexes of the aforementioned rats were below 0.300 and may be classified as non-obese or normal rats based on the definition of Malafaia [47] while the rats in HFD control had Lee’s index of >0.300 and may be classified as obese. The ability of the extracts to reduce body weight, adiposity, BMI and Lee’s index of rats could be due to the abundance of phenolic compounds and flavonoids in the plants [16]. These phytochemicals exert anti-obesity property by inhibiting adipogenesis [17].
This study showed that administration of C. albidum and I. gabonensis extracts did not affect food intake, and was consistent with findings from the orlistat®-treated group. The extracts probably produced anti-obesity effects, in high fat diet (HFD) induced obese rat model, without affecting the appetite. Similar results were reported by Lim et al. [49] where plant extracts did not alter the appetite of experimental obese rats, yet demonstrated anti-obesity effect.
Liver abnormality is often associated with obesity and hyperlipidaemia [50]. Obesity has a hepato-degenerative effect by inducing steatosis and oxidative damage to cells [51]. This may also explain the observed significant (p < 0.05) increase in the liver function parameters (AST, ALT, ALP, total and conjugated bilirubin) of the HFD control in comparison with the extracts and orlistat® treated groups. Similar results were obtained by Azubike et al. [52] in which treatment of obese rats with plant extract restored normal liver function parameters
High levels of urea and creatinine are suggestive of impaired kidney function. Obesity is a strong risk factor for onset of kidney disease. In obese people, hyper-filtration takes place to fulfil the increased metabolic needs of excess body weight. The increase in intra-glomerular pressure can increase the risk of developing chronic kidney disease [53]. A similar scenario might have caused the significant (p < 0.05) increase in serum urea and creatinine levels in the HFD group in the present study. Nevertheless, administration of the extracts or orlistat® resulted in an ameliorative effect against kidney inflammation and damage induced by high fat diet due to its ability to treat obesity.
It is a well-known fact that obesity and hyperlipidaemia are important risk factors for the development of cardiovascular diseases [54]. Serum creatine kinase (CK) and lactate dehydrogenase (LDH) activities were employed as indicators of cardiovascular dysfunction in this study. The observed significant (p < 0.05) reduction in serum creatine kinase and lactate dehydrogenase activities in the rats treated with the extracts when compared with the HFD control suggests the protective effects of the extracts against obesity induced cardiovascular stress or injury in the experimental rats. Similar findings were reported by Poongkodi et al. [55].
Histopathological evaluation of the liver and kidney tissues of HFD control revealed inflammation of the liver and kidney tissues. These histopathological findings validate the results obtained in the biochemical assays in the assessment of kidney and liver functions which indicated high activities of liver function enzymes, creatinine and urea levels as evidence of injury to the liver and kidney in rats from HFD control. On the other hand, treatment of the obese rats with the extracts of C. albidum and I. gabonensis; reference anti-obesity drug, orlistat® caused complete reversal of the damage to the organs with comparable features to that of the rats fed on the normal fat diet (NFD control).
5. Conclusion
From the results obtained, it can be concluded that leaf extracts of C. albidum, I. gabonensis possess significant anti-obesity and anti-hyperlipidaemic properties that are analogous to that of the reference anti-obesity drug (orlistat®). These effects may be due to the abundance of phenolic compounds, saponins, flavonoids, alkaloids and tannins in the plants. Comparatively, I. gabonensis extract was more effective in ameliorating obesity than the extract of C. albidum. These observations support and encourage further evaluation of C. albidum and I. gabonensis leaf extracts as an alternative therapeutic remedy for obesity.
Acknowledgements
The authors acknowledge the members of Malaria Research, Molecular Biology and Toxicology Unit, Department of Biochemistry, Faculty of Life Sciences, University of Benin for their support.