1. Introduction
Obesity is a critical topic in public health and preventive medicine and recognized as a 21st century pandemic [1]. Overweight and obesity increase the risk of hypertension, type-2 diabetes, CHD [1], cancer [2] and dementia [3], and reduce life expectancy [4].
With progressive weight gain there is an initial increase in adipocyte size and subsequently numbers. When existing adipocytes are replete with fat, the concerted action of extracellular signals and intrinsic transcription factors and co-activators triggers mitotic clonal expansion of fibroblastic pre-adipocytes which subsequently differentiate into immature and finally mature adipocytes [5]. Current research focuses on ways to increase lipolysis and fat oxidation, and to reduce lipogenesis, adipocyte size, and the differentiation and proliferation of pre-adipocytes [6-8].
Following weight loss, adipocyte size is reduced by lipolysis but adipocyte numbers are maintained, facilitating subsequent weight regain. Since degree of obesity is linked to adipocyte numbers, inhibiting differentiation and proliferation of pre-adipocytes is an interesting therapeutic strategy [6,7]. Botanical drugs appear promising. They may be safer (if derived from food plants) than pharmaceuticals [9-11], as evidenced by the documented pharmacology of Hoodia [12] and capsiate [13].
Caralluma fimbriata (C. fimbriata), an edible succulent belonging to the family Asclepiadaceae, grows wild in India, Africa, and Europe [14] and was cultivated in Britain as far back as 1830 [15]. Listed by the Indian Health Ministry as a vegetable and famine food, it is widely consumed as a food, appetite suppressant and treatment for diabetes [14]. A standardised extract of C. fimbriata containing pregnane and metastigmane glycosides [16] is marketed as a supplement for weight loss. There are no reports of adverse effects [17] and an acute oral toxicity study on rats showed no toxicity at doses up to 5 g/kg (Kurpad et al., unpublished).
Appetite suppressing effects of C. fimbriata have been documented [18]. Two unpublished reports of doubleblind, randomized, placebo-controlled trials substantiate the weight reducing property of C. fimbriata. More recently, an animal study [19] showed its ability to prevent adiposity induced by a cafeteria diet. This paper reports the anti-adipogenic property of C. fimbriata in the mouse-derived 3T3-L1 pre-adipocyte cell line, an accepted model for the in vitro evaluation of anti-obesity drugs, using cytoplasmic/nuclear localization of cyclin D1 as the molecular marker [6,8,20].
2. Materials and Methods
2.1. 3T3-L1 Cell Culture
3T3-L1 mouse embryo fibroblasts, obtained from the NCCS, Pune, India, were cultured as described elsewhere [21]. Cells were cultured in Dulbecco’s minimal essential medium (DMEM) with 10% fetal bovine serum at 37˚C in a humidified atmosphere with 95% air and 5% CO2. All experiments were carried out using cells within passage 20, in quadruplicate.
2.2. Test Material
Caralluma fimbriata extract (CFE), the aqueous fraction of a methanolic extract of the aerial parts, was obtained from GreenChem, Bangalore, India. Pregnane glycosides (the putative therapeutic compound) [22] were standardized to 25%. A stock solution of this extract was prepared at 1mg per ml concentration in culture medium and subsequently diluted in culture medium to obtain test concentrations from 20 to 500 ug/ml. Medium was the negative control, 10 ug/ml hydroxyurea (Sigma Chemical Co), a known inhibitor of cell division was the positive control.
2.3. Inhibition of 3T3-L1 Cell Growth by CFE (25%) - Cell Count
One hundred cells were seeded per well in a 12 or 24 well plate. Cells in each well were counted after 24 hr to check plating efficiency, giving the initial count. Medium was discarded and replaced with fresh medium containing the test drug at 100 to 500 μg/ml and 20 to 100 μg/ml concentrations, the positive control and the negative control. Cells were re-counted after 12, 24, 36 and 48 hr, using an inverted microscope (Axiovert, Carl Zeiss, Jena, Germany). Data for each time point and treatment were used to calculate the respective means ± standard deviations.
2.4. MTT Assay for Cell Viability
The cultured cells, treated with a single dose of the drugwere subjected to MTT assay [23]. 5000 cells were seeded per well in 96 well micro-titer plates and cultured for 24 hr. After discarding the medium, fresh medium containing negative control, positive control or the drug at 100 μg/ml concentrations was added and the cells incubated for 24 hr (8 replicates). The medium was then replaced with 20 µl of MTT (5 mg/ml). The plate was incubated for 5 - 6 hr in dark. Formazon crystals that developed were solubilized with 100 µl of dimethyl sulfoxide (DMSO) and color intensity read in an ELSIA reader (BioRad, CA, USA) at 570 nm, with 630 nm as the reference wavelength. The data were used to calculate the respective means ± standard deviations.
2.5. Immunofluorescent Assays for Cyclin D1
Localization of cyclin D1 was carried out using mouse anti-cyclin D1 monoclonal antibody (Santa Cruz Biotechnology Inc., CA, USA). Treatment procedures were the same as described for the MTT assay.
2.6. Fluorescent Microscopy
The treated cells were washed thrice with PBS (pH-7.4), fixed in 1% formalin in PBS for 2 min and air-dried. After three subsequent washes in PBS, the cells were incubated with 10 % blocking serum for 20 min and washed again in PBS. The cells were incubated for 60 min with the primary antibody for cyclin D1 [(72-13G): sc-450, mouse monoclonal antibody, dilution range 1:100] in PBS, with 1.5% blocking serum. After three washes in PBS for 5 min each, the cells were incubated with fluorochrome-conjugated secondary antibody [goat anti-mouse IgG-FITC: sc-2010 (dilution range: 1:100-1:400)] in PBS with 1.5% - 3% normal blocking serum, in a dark chamber for 45 min. After three washes in PBS, the cells were loaded on to a cover slip, mounted in 90% glycerol in PBS and examined in a fluorescent microscope (Axioscop 2 Plus, Carl Zeiss, Jena, Germany) with appropriate filters (wavelength 450 - 490 nm).
2.7. Indirect Immunofluorescent Assay
Cells were grown in 8-well chamber slides and treated with CFE at 100 ug/ml concentration, hydroxyurea at 10 ug/ml concentration or left untreated. The cells were then fixed with 10% buffered formalin and permeabilized with chilled acetone (histological grade, EMD Biosciences, San Diego, CA). The fixed cells were then blocked with goat serum and incubated with the primary antibody for cyclin D1 [(72-13G): sc-450, mouse monoclonal antibody, Santa Cruz, CA, USA, dilution range 1:100) in a humidified chamber at 37˚C. After incubation with the primary antibody, the cells were washed with PBS and conjugated with secondary antibody with the fluorescent dye Alexa Fluor 594 (red) (Molecular Probes Inc., Oregon, USA). The chambers were removed, the slides air-dried, and the nuclei stained with 4’-6-Diamidino-2- phenylindole hydrochloride (DAPI) by mounting the slide with Vecta shield mounting medium containing DAPI (Vector Laboratories Inc., CA, USA). Localisation of cyclin D1 was observed in a confocal microscope using UV lasers (Leica, Jena, Germany).
2.8. Analysis of Data
All the values were expressed as the mean ± SEM and analyzed adopting one way analysis of variance (ANOVA).
3. Results
3.1. Inhibition of 3T3-L1 Cell Growth by CFE
The 3T3-L1 cells were treated with CFE at increasing concentrations from 20 to 500 μg/ml for 12, 24, 36 and 48 hr, with hydroxyurea as positive control and untreated as negative control. Negative control 3T3-L1 cells exhibited normal growth. Hydroxyurea-treated positive control cells were inhibited in division beyond 12 hr of treatment and the cell counts decreased, indicating cell death. In cells treated with CFE, cell growth was inhibited with all concentrations of the drug, in a doseand time-dependent manner. At 100 ug/ml and above, cell growth was inhibited significantly and there was a delayed but dose-related reduction in absolute cell counts, indicating cell death.
This is presented in Tables 1 and 2. Data are presented as mean ± SEM. At zero hr, there were no significant differences between and within the groups (P < 0.05); as time progressed statistically significant differences were noted between the negative and positive controls (a) and between negative control and CFE-treated groups (b) (P < 0.05) at all concentrations.
3.2. Loss of Viability of 3T3-L1 Cells on Treatment with CFEMTT Assay
Treatment of 3T3-L1 cells with CFE at 100 μg/ml concentration resulted in loss of viability of the cells and the effect was dependent on duration of treatment.
3.3. Inhibition of Nuclear Import of Cyclin-D1 by CFEDirect Immunofluorescent Assay
Cyclin D1 was immunolocalized in untreated and hydroxyureaand CFE-treated 3T3-L1 preadipocyte cells using specific antibody and observed in a fluorescent microscope. In the untreated cells cyclin D1 was local-

Table 1. Inhibition of 3T3-L1 cell growth by CFE at 100 to 500 μg/ml.

Table 2. Inhibition of 3T3-L1 cell growth by CFE 20 to 100 μg/ml, as revealed in the manual cell count.
ized both in the nucleus and cytoplasm (see Figure 1). In the hydroxyurea-treated cells, cyclin D1 was mostly localized in the cytoplasm with little localization in the nucleus (see Figure 2). The result was similar in cells treated with CFE (see Figure 3).

Figure 1. The direct immunofluorescent assay for cyclin D1.
3.4. Inhibition of Nuclear Import of Cyclin D1 by CFEIndirect Immunofluorescent Assay
In order to acquire additional evidence for inhibition of nuclear import of cyclin D1 by CFE treatment, the cells were subjected to indirect immunofluorescent assay and observed in a confocal microscope. In untreated cells cyclin D1 was localized in the nucleus and cytoplasm. When DAPI pseudo-stained nuclei were overlaid the nuclei fluoresced orange, confirming nuclear localization of cyclin D1 (See Figure 4, upper panel). In hydroxyurea-treated cells most nuclei appeared empty of cyclin D1, and in the overlay most nuclei fluoresced bright red (See Figure 4, middle panel). Treatment with CFE (100 μg/ml) produced a similar result, confirming little localization of cyclin D1 in the nuclei of most cells (See Figure 4, lower panel).
4. Discussion
The initial phase of our study showed that a Caralluma fimbriata extract standardized to pregnane glycosides inhibits proliferation and at high concentrations impairs the viability of 3T3-L1 pre-adipocytes. These effects are generally doseand duration-dependent and the optimal dose is at, or near, 100 μg/ml. The ability of CFE to inhibit the proliferation of pre-adipocytes is similar to the effects of other compounds on 3T3-L1 cells, such as dehydroepiandrosterone (DHEA) [24], conjugated linoleic acid (CLA) [25], (-)-epigallocatechin gallate [7], esculetin [8] and phenolic acids [6]. The second phase of our work revealed that CFE inhibits the import of cyclin D1

Figure 2. The direct immunofluorescent assay for cyclin D1.

Figure 3. The direct immunofluorescent assay for cyclin D1.
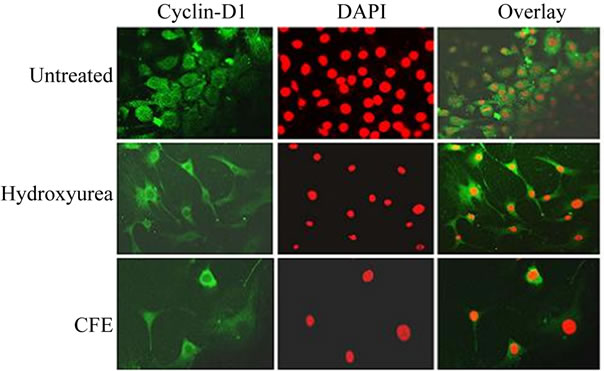
Figure 4. Indirect immunofluorescent assay for cyclin D1.
from the cytosol into the nucleus, thereby arresting the cell cycle at G1 phase. This is the first report of the antiadipogenic mechanism of action of C. fimbriata.
The cell cycle is a complex process driven by sequential activation of cyclin-dependent kinases (CDKs) in association with regulatory subunits (cyclins) during G1-S and G2-M of the cell cycle [26]. Cyclins are proteins named after their cyclical expression and degradation through ubiquitination during the cell cycle[27]. CDKs are negatively regulated by cyclin-dependent kinase inhibitors (CKIs) including p16 INK4A, P21CIP1, P27KIP1, etc., [28]. Members of the p21 family proteins inhibit CDK activity by forming a ternary p21 -cyclin D-CDK4 complex, whereas members of the INK4 family specifically inactivate CDK4 activity by forming a binary INK4-CDK4 complex. Within the CDK family, CDK4/6 is unique in that it is regulated by both the CIP/KIP and lNK4 families of inhibitors. This regulation provides CDK4/6 the ability to serve as integrators for the convergence of cell growth control signals from different stimulatory and inhibitory regulation pathways. This suggests that different cell growth control signals, such as terminal cell differentiation, may act on the cell cycle by regulating CDK4/6, pointing to the critical importance of CDK4/6 and the associated regulatory proteins [28].
G1 progression and S-phase transition are key events in the cell cycle; mitogenic factors input here, and there is the G1-S check point. Once this check point is passed, DNA synthesis and replication cannot be stopped. The D-type cyclins are regarded as sensors of the extracelluar matrix, which link mitogenic stimuli to events in the cell cycle [29]. CDK4/6-cyclin D complex couples extracellular growth signals with the cell cycle [28]. Cyclin D1 is an unstable protein found at high levels at G1 phase and its induction does not require de novo mRNA synthesis [30,31]. In mammalian cells CDK4 or CDK6, in combination with D-type cyclins, plays key roles in regulating G1 progression [28]. Cyclin D1 is highly expressed in several cancers [32]. Over-expression of cyclin D1 can produce oncogenic transformation of cells both in vitro and in vivo [33]. Over-expression of cyclin D1 is observed in most dividing cells particularly in a variety of carcinomas [32]. Inappropriate accumulation of cyclin D1 in the sub-cellular compartments may lead to either cell survival or cell death [33]. Liu et al. [34] suggested that cyclin D1 may be a potential therapeutic target for malignancy, leading to studies focusing on cell cycle proteins targeting, particularly, the multiple cyclin-CDK holo-enzymes or those specifically inhibiting cyclin D1-CDK4/6 [35]. The possible mechanism of inhibition of 3T3-L1 pre-adipocyte proliferation by CFE during an early phase of adipogenesis would involve either downregulation of CDK or inhibition of import of cyclin D1- CDK holo-enzyme from the cytosol into the nucleus, either through up-regulation of CKIs p16INK4a, p15INK4b, p27 and/or CDC25 via SMAD3/4 through TGF-β during the G1-S phase transition or independently of these molecules [7,27,28,36] by one or more of the compounds present in C. fimbriata, resulting in G1 arrest.
Our immunofluorescent analyses reveal that that cyclin D1 is localized both in cytosol and nucleus in the negative control pre-adipocyte cells. However, pre-adipocyte cells treated with CFE exhibited predominant cytosolic localization of cyclin D1. This indicates that CFE induces G1 arrest in the pre-adipocyte cells by preventing import of cyclin D1 into the nucleus. This implies that on treatment with CFE, CDK 4/6 is not imported from the cytosol to the nucleus. CDK4/6 phosphorylates RB (retinoblastoma tumor suppressor) protein so as to release E2F transcription factor from RB-E2F binding to facilitate the transcription of genes necessary for S-phase DNA synthesis [35]. Considering the critical role played by cyclin D1 in pre-adipocyte proliferation, recent studies have focused on developing agents to target cyclin D1 and induce G1 arrest in preadipocytes. The discoveries that pre-adipocyte cells treated with (-) epigallocatechin decreased cyclin D1 expression, which inhibited the proliferation through G1 arrest [7] and proliferation of rat mesangial cell was prevented by YC-1 (1-benzyl-3-(5’-hydroxymethyl-2’-furyl) indazole through decreased expression of cyclin D1 and CDK 4 [37] support our inference in this regard. They also suggest that CFE may have anti-cancer properties.
The results suggest that CFE merits further research and clinical development. Its ability to inhibit adipogenesis in cafeteria-fed animals [19] and weight gain in humans [18] indicates that our in vitro findings are biologically and clinically relevant, and match CFE’s ethnobotanical background.
5. Conclusions
The initial phase of our study showed that CFE standardized to pregnane glycosides inhibits proliferation and at high concentrations impairs the viability of 3T3-L1 pre-adipocytes. These effects are generally doseand duration-dependent and the optimal dose is at, or near, 100μg/ml. CFE’s ability to inhibit the import of cyclin D1-CDK complex into the nucleus may be due to down-regulation of CDK 4/6 or maintenance of CKIs at default. We are investigating this, and are extending this work by studying the impact of CFE on primary mouse pre-adipocytes differentiating in culture, using pure pregnane and metastigmane glycosides.
6. Acknowledgements
The research facilities made available under the FIST program of DST, Government of India, New Delhi (No. SR/FST/LSI-112/2002) and Special Assistance ProgramDepartment Research Support (SAP-DRS) of University Grants Commission, Government of India, New Delhi (No.F.3-5/2007) to the Department of Animal Science, Bharathidasan University, Tiruchirappalli, India, are gratefully acknowledged.
7. Declaration of Interest
Ramaswamy Rajendran is employed by GreenChem, an industrial group that manufactures plant extracts. GreenChem supplied Caralluma fimbriata extracts used in this study. Ramasamy Venkatesh is employed by GenCor Pacific, which first developed the CFE raw material. Paul Clayton provides occasional consultancy services to GenCor Pacific. The other authors have no vested commercial or other conflicts of interest.