Assessment of the Quality and Shelf-Life in Enriched n3 PUFA Raw Beef Patties Using Dry Soybean Sprouts as Antioxidant ()
1. Introduction
The appearance of fresh retail meat is a major determinant of its appeal to consumers and, consequently, sales of the product. Ground beef retail display oxidizes at a faster rate than whole muscle products, and this oxidation causes a brown discoloration on the product which is discriminated by consumers [1] . Oxidation also results in the development of detrimental off-flavors and odors; thus, prevention of oxidation in ground beef has the potential to lengthen the shelf life and improve the profitability of ground beef products [2] . Complex mechanisms and reaction processes are involved in lipid oxidation, and it is generally accepted that oxidation occur mainly via a radical chain reaction including initiation, propagation, and termination stages [3] [4] . One effective method for slowing oxidation is to use antioxidants to break the radical chain reaction, thereby keeping the nutriational and sensory qualities of meat for longer.
Currently, polyunsaturated n3 fatty acids have been implicated as critical nutrients for human health, and fortification of foods with n3 polyunsaturated fatty acids (PUFA) is an emerging area of commercial and academic interest. Therefore, different alternatives were studied to improve lipid profile in meat products, and to regain of the consumer trust in these products. Several investigators [5] -[7] have reported improved fatty acid profile in muscle foods by the addition of n3 PUFA from vegetable oils such as soybean oil. However, incorporation of these fatty acids into food systems is potentially problematic due to their propensity to readily oxidize.
Compared with synthetic antioxidants, natural antioxidants are of great interest because of their safety and health characteristics. Most natural antioxidants are obtained from plant resources including culinary herbs, spices, fruits, vegetables, and oilseed products [4] . Soybean is a complex matrix with several bioactive compounds, including peptides and proteins, isoflavones, saponins, and other compounds with antioxidant properties [8] . The majority processed soybean products have been derived from dry mature soybeans. Although germination processes have been developed to overcome the disadvantages of soybean seed used in food products [9] , it also was observed to increase their antioxidant properties. At present, germinated soybean products used for human consumption are the soybean sprouts which are commercialized as raw staple or minimally processed vegetables [10] . The development of functional foods from soybean sprouts as meat ingredients opens up new strategies to produce healthier foods.
Therefore, our objectives in this study were 1) to evaluate the antioxidant properties of dry soybeans sprouts from Glycine max (L.) Merr. (MUNASQA®); and 2) to evaluate nutritional composition and fatty acid profile of meat patties with partial replacement of fat by soybean oil and the addition dried soybean sprouts and 3) to asses color changes and the effectiveness of this element in preventing or reducing lipid oxidation of fortified raw patties under frozen storage. Microbiological characteristics of the products were also evaluated.
2. Materials and Methods
2.1. Dry Soybean Sprouts Preparation
Dry soybean sprouts (DSS) were obtained from previously selected soybeans (Glycine max (L.) Merr., MUNASQA®, Tucumán, Argentina), selection was made based on shape and size. The beans were soaked in water for one hour at 25˚C and then germinated in darkness at 30˚C in a controlled temperature chamber. Once the sprouts reached 5 cm of length approximately they were separated from the beans, washed in sterile water and dehydrated at 40˚C in a static drying chamber until 89% dry weight. Then dry soybean sprouts were ground to a powder.
2.2. Preparation of Hydrophilic and Hydro Alcoholic Extracts
Two grams of dry soybean sprouts were ground in a pepper grinder and extracted overnight at 4˚C with 40 mL of 50 mmol tris-HCl buffer (pH 8.0) containing 1 mmol ethylenediaminetetraacetic acid (EDTA, Merck-KGaA, Darmstadt, Germany) to obtain a higher enzymatic concentration. The resulting hydrophilic crude extract was centrifuged in a refrigerated centrifuge (Rolco* CM 2036, Aparatos para Médicos Laboratorios e Industrias, Buenos Aires, Argentina) at 1000 × g for 20 min. The upper layer obtained after centrifugation was used for superoxide dismutase assay.
At the same time, 2 g of dried and ground soybean sprouts were extracted twice with a mixture of ethanol:water (70:30). The mixture was centrifuged for 20 minutes and the supernatant was dried under vacuum (50˚C and 4000 Pa) to obtain a dry extract, which was used for total phenols, flavonoids and reducing power determinations, DPPH and ABTS assays, and measurement of metal chelating activity.
Notions of the experiment: DPPH:1,1-diphenyl-2-picrylhydrazyl and ABTS: 2,2’-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid).
2.3. Antioxidant Properties
2.3.1. Superoxide Dismutase Assay (SOD)
This SOD assay is routinely used for the measurement of superoxide dismutase in barley. The method is based on the inhibition of the reduction of cytochrome c by superoxide dismutase enzyme when the superoxide radical anions are generated by a xanthine/xanthine oxidase system [11] . SOD E.C. 1.15.1.1 from Bacillus stearothermophilus was used as a standard (Sigma-Aldrich, St Louis, MO, United States)
One centimeter light path glass cuvettes were used containing 150 μmol of potassium phosphate, pH 7.8; 0.3 μmol EDTA; 0.03 μmol ferricytochrome c; 0.15 μmol xanthine; 10 μL xanthine oxidase (0.65 units∙mg−1 proteins) ; 10 μL hydrophilic crude extract without dilution and water to a total volume 3 ml. The mixtures were incubated at 25˚C for 2 min without xanthine, which was then added to start the reaction. The reduction of cytochrome c was followed by recording the increase in absorbance at 550 nm over a period of 3 min, using a Beckman DU® 640B spectrophotometer (Fullerton, CA, United States). One unit of superoxide dismutase is defined as the amount that lessens the rate of reduction of cytochrome c by 50%.
2.3.2. Total Phenolic Compounds Determination
Total phenolics content was determined using Folin-Ciocalteau [12] . One milliliter of diluted dry extract or a standard solution (1 ml) was added to 10 ml deionised water and 2 ml Folin-Ciocalteau reagent (Merck, Darmstadt, Germany). The mixture was then allowed to stand for 5 minutes and 2 ml of sodium carbonate were added (saturated solution). After 1 hour in darkness, the absorbance at 735 nm was measured. The concentration of total phenolics was expressed as mg of gallic acid equivalent per g of dry extract by using an equation that was obtained from a standard gallic acid graph (R2 0.995).
2.3.3. Flavonoids Determination
Total flavonoids were measured by a colorimetric assay according to Kim et al. [13] with some modification. To 4 ml H2O in a 10 ml volumetric flask was added 1 ml of sample:water (50:50, v/v) or standard solutions of quercetin (0 - 500 mg∙L−1, Sigma-Aldrich, St Louis, MO, United States). At zero time, 0.3 ml of 50 g∙L−1 NaNO2 was added to the flask. After 5 min, 0.3 ml AlCl3 (100 g∙L−1) was added. At 6 min, 2 ml of 1 mol∙L−1 NaOH were added to the mixture and immediately diluted with 2.4 ml of H2O. Absorbance of the mixture was read at 510 nm versus water blank. Total flavonoids were expressed as mg quercetin equivalents g−1 of dry matter.
2.3.4. Determination of Vitamin C Content
The content of vitamin C was determined by the titration procedure. Aliquots of 0.1 ml of the extract were diluted in 20 ml of oxalic acid (2 g∙L−1) for titration with 0.4 g∙L−1 dichlorophenol-indophenol (Anedra, San Fernando, Argentina). The ascorbic acid concentration was calculated by comparison with L(+)-ascorbic acid standard (Merck-KGaA, Darmstadt, Germany).
2.3.5. Reducing Power Determination
The reducing power was determinate as described by Oyaizu [14] . Various dilutions of the previously obtained extract (0.01:0.99; 0.02:0.98; 0.05:0.95; 0.1:0.90; v/v) were mixed with 2.5 ml of 200 mM sodium phosphate buffer (pH 6.6) and 2.5 ml of 10 g∙L−1 potassium ferricyanide (Sigma-Aldrich, St Louis, MO, United States). The reaction mixture was incubated in a temperature-controlled water bath at 50˚C for 20 minutes, followed by the addition of 1 ml of 10% trichloroacetic acid. The mixture then was centrifuged and the supernatant obtained
(1 ml) was treated with 1 ml distilled water and 200 μl of 0.1% FeCl3. The absorbance of the reaction mixture was measured at 700 nm in a spectrophotometer. Higher absorbance indicates a higher reducing power.
2.3.6. Scavenging Activity on DPPH and ABTS Radicals
The radical scavenging ability of crude extract was determined at different concentrations in an ethanolic DPPH solution (9 × 10−3 g 100 ml−1, Sigma-Aldrich, St Louis, MO, United States). 100 ul of hydrophilic extract at various dilutions with water (0.01:0.99; 0.02:0.98; 0.05:0.95; 0.1:0.90, v/v) were added to three milliliters of ethanolic solution of DPPH. The decrease of absorbance at 517 nm was continuously recorded in a UV-Vis spectrophotometer for 10 minutes at room temperature.
The radical scavenging activity of the ABTS radical cation was measured as the informed by Re et al. [15] . The bleaching rate of ABTS in the presence of the sample was spectrophotometrically monitored at 734 nm during 10 minutes and the reaction was started by adding 100 μl of antioxidant solutions.
The inhibition percentage of free radicals DPPH and ABTS (I %) was calculated using the following equation [16] :

Furthermore, radical scavenging activities for both determinations were expressed as mg of Vitamin C equivalent per g of sample through the dose-response curves for Vitamin C.
2.3.7. Measurement of Metal Chelating Activity
The 2,2’-bipyridyl competition assay was conducted to measure the Fe2+ chelating activity of the samples as the indicated by Zhou et al. [17] . The reaction mixture contained 0.1 mL of 1 mM FeSO4 solution, 50 uL of sample, 0.3 ml of 10% hydroxylamine-HCl, 0.4 ml of 2,2’-bipyridyl solution (0.1% in 0.2 M HCl), and 0.8 ml of TrisHCl buffer (pH 7.4). The absorbance at 522 nm was measured and used to determine Fe2+ chelating activity using EDTA as the reference standard and was expressed in mg EDTA equivalents per gram of dry extract.
2.4. Patties Preparation
Enriched beef patties were prepared using fresh lean beef meat and pork backfat obtained from local markets, and soy oil containing SFA 16 g/100g, MUFA 35 g/100g, PUFA n6 60 g/100g and n3 6 g/100g (Aceitera General Deheza S.A., Córdoba, Argentina) that was used as fat partial replacer.
Five different formulations were elaborated as follows: one control sample without addition of antioxidants (C) with beef and 20% of pork back fat (similar to commercial products); and four enriched beef patties (EP) with beef, 10% of pork back fat, 10% of soybean oil and with addition of DSS as natural antioxidant (0.5%, 1% and 2%), and 0.01% of butylated hydroxyanisole (BHA) as antioxidative positive control.
All formulations were shaped using Petri-dish (90 mm × 20 mm) to obtain 60 patties of approximately 100 g each one. The samples were packaged in oxygen permeable bags (2000 cm3/m2day) using a packing machine (RAPI-VAC S-750® SERVIVAC S.R.L., Buenos Aires, Argentina) stored at −18˚C and analyzed at 0, 30, 60 and 90 days.
Notions of the experimental samples: DSS: dry soybean sprout; C: control patty; EP: enriched patties; EP 0.5%: enriched patty with 0.5% of DSS; EP 1%: enriched patty with 1% of DSS; EP 2%: enriched patty with 2% of DSS; EP 0.01% BHA: enriched patty with 0.01% of BHA.
2.4.1. Proximate Composition
Moisture, protein, fat and ash contents were analyzed according to AOAC methods [18] . Total carbohydrates were quantified using Clegg anthrone methods [19] on an UV-Vis Beckman DU 640B® spectrophotometer (Fullerton, CA, United States).
2.4.2. Fatty Acids Composition
Total lipids were extracted as described by Bligh and Dyer [20] and fatty acid methyl esters were obtained according to AOAC methods [18] and analyzed using GC Mass Spectrometer (Thermo Fisher Scientific®, Austin, TX, USA) equipped with a 100% Cyanopropyl silicone capillary column (SPTM-2340 100 m, 0.32 mm IDfilm thickness 0.25 mm). The oven temperature was held at 140˚C for 5 min and subsequently increased at 4˚C/min to 220˚C. Injector temperature was 250˚C. Identification of fatty acid methyl esters was based on retention time of standard esters (Supelco® 37 Components FAME Mixture, Bellefonte, PA) eluting from the capillary column. Peak areas were integrated using chromatography data software, and concentrations of each ester were calculated as a percentage of the total area of the chromatogram.
2.4.3. Color Determination
Color measurements (L*, a* and b* values) were performed using an Evolution 600 UV-Vis (Thermo Scientific®, Austin, TX, USA) equipped with a integrate sphere using a VISIONlite colorCalc software to obtain CIE L*, a*, b* values. The L* value represents lightness and a* and b* values represent redness and yellowness, respectively. Color measurements of fresh patties were recorded on days 1 and 90 of storage.
2.4.4. Measurement of Lipid Oxidation
Lipid oxidation of beef patties was monitored by measuring the formation conjugated dienes (CD), peroxide value (PV) and thiobarbituric acid reactive substances (TBARS).
Conjugated dienes were determined as Aubourg [21] , pre-weighed lipid samples were diluted with hexane and the absorbance was measured at 233 nm in a Beckman DU® 640B spectrophotometer. The hydroperoxides conjugated dienes concentration was expressed in milliliter per milligram of lipids. The results were calculated as:

Where Bis the absorbance reading, V denotes the volume (ml) of the solvent and w is the mass (mg) of sample measured.
Determination of PV was conducted by the IDF method [22] . 0.01 g of samples were dissolved in 9.9 ml chloroform:methanol (70:30) solution and 0.05 ml of 30% (w/v) ammonium thiocyanate were added and mixed. Precisely 5 min after the addition of 0.05 ml of ferrous chloride in 3.5% v/v hydrochloride acid to the reaction mixture, the absorbance of the resulting red color was measured at 501 nm against solvent solution as blank. Results were expressed in meq O2/kg of lipids.
A modified thiobarbituric acid reactive substances (TBARS) method was used to evaluate the extent of lipid oxidation according to Ahn and Kim [23] . One hundred milligrams of lipids were taken, and the following reactives were sequentially added: 100 μl (BHA 36 g/l) and 2 ml of TBA/trichloroacetic acid (TCA) solution (20 mM TBA in 150 g/l TCA). The mixture was heated in a 90˚C water bath for 15 min and cooled at room temperature. Afterwards, two milliliters of chloroform were added and the mixture was centrifuged at 1.000 rpm for 15 min. The absorbance of the supernatant was measured at 532 nm in an UV-Vis Beckman DU 640B® spectrophotometer against a blank containing 0.1 ml H2O and 2 ml TBA/TCA solution. Malonaldehyde (MAD) standard curves were prepared by 1,1,3,3-tetramethoxypropane and TBARS were expressed as mg/kg of MAD equivalents of samples.
2.4.5. Antioxidant Activity
The antioxidative activity at the end of assay was expressed for each indicator as inhibition or reduction percent of peroxidation (RP%) with a control containing no antioxidant.

A higher percentage indicates a higher antioxidant activity.
2.5. Microbiological Analyses
Ten gram of each sample were homogenized with 90 ml of 0.1% peptone water and appropriate serial dilutions were plated in duplicate on plate count agar (PCA) at 37˚C for 48 h. Results were expressed as log number of colony forming units per g of sample (log cfu/g).
2.6. Statistical Analyses
The results were expressed as mean ± standard deviation. All statistical tests were performed using Statgraphics Plus for Windows software package. Data obtained from proximate composition, fatty acid profile, color measurement and oxidative stability parameters for each storage time (0, 15, 30, 60 and 90 days) were analyzed with one-way analysis of variance (ANOVA) using the formulations as factor. Differences were considered significant at p < 0.05 and the Fisher’s Multiple Range Tests were used for comparison of mean values.
All experiments and analyses were carried out in triplicate.
3. Results and Discussion
3.1. Antioxidant Properties of Dry Soybean Sprouts
Multifarious antioxidant systems are very important for protecting cellular membranes and organelles from the damaging effects of active oxygen species. These include antioxidant enzymes, no enzymatic antioxidants and secondary metabolites [24] . Also, the antioxidant reactivity characterizes the starting dynamics of antioxidation at certain concentration of an antioxidant or antioxidant mixture [25] . Table 1 shows a summary of the antioxidant properties of DSS.
SOD activity in crude extract from dry soybeans sprouts was higher than soya bean (640 U∙g−1), wheat flour (56 U∙g−1) and commercial ale malts (170 U∙g−1) according to reported by Bamforth [11] . This value also is higher than the superoxide dismutase from garlic (20.6 U∙g−1) [26] , and those in selected Indian medicinal plants [24] . However this value is lower than the values informed in dry soybeans sprouts from NIDERA® Glycine max (L) Merr. (3110 U/g) [27] .
The antioxidant properties measured as phenolics compounds content, flavonoids content, vitamin C content and valued as DPPH and ABTS radicals scavenging activity and reducing power, would allow estimating the antioxidant capacity of vegetable natural extracts. The phenolics content of hydroalcoholic extract from soya sprouts and flavonoids content were higher than those reported in aqueous extracts from rosemary, roasted Arabica coffee and grape skin which ranged between 67 and 198 mg phenol equivalents/g [28] , and that the informed in 10 edible plants whose values were from 3.13 to 72.30 mg gallic acid/g [29] . The vitamin C content of dry soya sprouts was high in contrast with other investigations [27] [30] which reported that dry soya bean seed did not contain vitamin C, but after 3 days of germination the vitamin C level reached 250 mg∙Kg−1 dry matter, therefore the value found for this variety of soybeans is higher than the informed by this author.
Relating to reducing power, similar results was reported before [27] indicating that soya bean without fermentation did not contain reductones, chemical substances responsible of reducing power.
Free radical-scavenging is one of the known mechanisms by which antioxidants inhibit the lipid oxidation [31] . The DPPH assay is one of the most widely employed and preferred methods for measuring the radical scavenging activity of plant extracts. This assay is rapid, relatively simple, and easily standardized [32] . At the same time the ABTS radical assay determines the single electron-transfer capabilities of these [15] . In the present study the DPPH and ABTS radicals scavenging capacity of the dry soybean sprout extracts was elevated, probably due to the high phenolic and flavonoids content in this ingredient.

Table 1. Antioxidant properties of dry soybean sprout extracts.
Antioxidants compounds and antioxidant activity of DSS extracts.
Also was determinate that DSS extract presents 43.2 ± 2.1 mg EDTA equivalents/g of metal chelating activity. Transition metals are known to act as catalysts of the oxidation of lipids; therefore DSS would have great effect in retarding lipid peroxidation and subsequently prevention rancidity of foods due to the chelating ability of Fe2+.
3.2. Nutritional Value and Lipidic Profile of Enriched Beef Patties
Proximate composition of DSS and enriched burgers with soybean oil as fat partial replacer and different concentrations of the additive are shown in Table 2.
Since dehydrated soybean products are not commercialized as additives or similar products, due to the degree of processing they have, the comparison of the nutritional properties can be done with other products from soy as flour and isolated protein. The protein, carbohydrate and ashes contents of DSS are in concordance with the values found for different soybean flours samples, while the values found for moisture and fat are lower than the informed by Ndife et al. [33] and Eze et al. [34] . These results shows the potentials use of dry soybean sprouts, which is rich in protein of high biological value and have not been adequately exploited in manufacture of different types of food products.
Moisture analysis of patties showed significant differences (p < 0.05) between formulations, the addition of DSS decreased moisture content in all samples. This is in agreement with other researchers [35] -[37] who reported a decrease in moisture content of beef patties formulated with oat flour, bambara groundnut and Wakame, probably because of increased solid contents.
The higher concentration of DSS, the higher carbohydrate content of raw patties (p < 0.05), which could be due to the high content of carbohydrates in soybean sprouts.
The differences in protein content and fat content between formulations were found to be significant (p < 0.05). The effect of DSS concentration however was found to be non-significant; although the protein and fat contents at zero DSS concentration were higher than the enriched DSS patties. Fat content of the raw patties decreased when the levels of DSS were increased, which is in concordance with other reports [36] .
Ashes analysis of all samples did not show significant differences between formulations (p < 0.05).
Five fatty acids composed by palmitic (21.50 ± 0.14), oleic (9.65 ± 0.05), linoleic (37.60 ± 0.15), linolenic (12.47 ± 0.004) and docosaenoic (4.14 ± 0.16) comprised around 85% of total fatty acids in DSS analyzed samples. The fatty acid compositions of raw patties manufactured with soybean oil as replacer of pork back-fat are shown in Table 3.
Six fatty acids, namely palmitic, stearic, oleic, linoleic, linolenic and CLA (10c, 12c) acids, comprised around 97% of total fatty acids in analyzed meat products. As expected, the treatment with vegetable oil had a significant effect on the fatty acid composition of these products. The addition of the vegetable oils to burger patties significantly reduced the content of palmitic and stearic fatty acids, and therefore, SFA were decreased compared to the control counterparts. Also n3 PUFA of short chain content increased from 1.65% to 4.50%, and n6 PUFA from 21.32% to 40.87%. The PUFA/SFA relationship increased around three times, and the n6/n3 ratio was maintained below 10.
The fatty acid composition of meat foods has a great impact on the nutritional value, oxidative stability and sensory properties [38] . Products obtained in this way (including 10% of soybean oil in the recipe of porcine

Table 2. Mean values of proximate composition of dry soybean sprouts and enriched raw patties (EP) formulated with different concentrations of DSS.
Results are presented as means ± standard deviation of three replicates. Different letters (a) in the same column denote significant differences among formulations (p < 0.05). DSS: dry soybean sprout; C: control patty; EP: enriched patties; EP 0.5%: enriched patty with 0.5% of DSS; EP 1%: enriched patty with 1% of DSS; EP 2%: enriched patty with 2% of DSS; EP 0.01% BHA: enriched patty with 0.01% of BHA.

Table 3. Fatty acid profile of enriched patties formulated with different concentrations of DSS in g/100g dry product.
Results are presented as means ± standard deviation. Means in the same row with different letters are significantly different among formulations (p < 0.05). -: not detected. C: control patty; EP: enriched patties; EP 0.5%: enriched patty with 0.5% of DSS; EP 1%: enriched patty with 1% of DSS; EP 2%: enriched patty with 2% of DSS; EP 0.01% BHA: enriched patty with 0.01% of BHA. SFA: Saturated Fatty Acids, MUFA: Monounsaturated Fatty Acids, PUFA: Polyunsaturated Fatty Acids. *Atherogenic Index calculated as follows: (C12:0 + 4 × C14:0 + C16:0)/(ΣMUFA + ΣPUFA).
burger patties) were healthier due to the higher contents of essential fatty acids and due to the reduction of the atherogenic potential of these meat products [39] [40] .
3.3. Color Changes and Oxidative Stability of Enriched Beef Patties
The color of meat and meat products after slaughter and manufacturing is altered by increased metmyoglobin [41] and by the addition of ingredients or non-meat substances [42] [43] .
Formulation and frozen storage affected color parameters (p < 0.05) in some samples as can be observed in Table 4.
Addition of soybean oil affected color parameters compared to the control (p < 0.05) at the beginning of storage. The addition of DSS generally had little effect on color compared to EP 0.01% BHA (p < 0.05), this effect could be attributed to the decrease of meat content in the formulation; however significant changes were observed at the end of storage (p > 0.05). The a* value (redness) is the most important color parameter in evaluating meat oxidation, a decrease in redness makes to the meat product unacceptable to consumers [44] . In all samples a* value decreased with frozen storage but the redness of the control samples descended rapidly and at the end of storage was significantly lower (p < 0.05) than that of the antioxidant-containing meat products. Dry soybean sprouts incorporated in the patties are potentially useful in prolonging the shelf life of the meat products by preserving of the red color. The results found for color changes are in concordance with the informed by another’s researchers [28] [45] [46] in different raw products added with natural ingredients as antioxidants.
Unsaturated fatty acid double bonds occur naturally as methylene interrupted systems. When meat begins to oxidize, the double bonds of these fatty acids shift to create conjugated systems (CD), which can then be measured spectrophotometrically [47] . PV measures the amount of hydroperoxides formed as primary oxidation products at the initial stage of oxidation [47] . These peroxides, however, are very reactive and may actually decrease during the storage of lipid-containing foods [48] . Different concentrations of the natural antioxidant had a

Table 4. Color parameters of raw enriched patties added with different concentrations of DSS.
Results are presented as means ± standard deviation. AMeans within a column with different letters are significantly different (p < 0.05). aMeans within a row with different letters are significantly different (p < 0.05).
significant effect on the lipid oxidation of samples (p < 0.05), as can be observed in Figure 1. The control treatment had significantly higher PV and TBARS values (p < 0.05) compared to samples with antioxidants at the end of storage (Figure 1), except for CD having EP 2% DSS the highest value. Fresh meat products can be considered well preserved in regards to oxidative changes [49] , when they had less than 3 mg MDA/kg sample.
In relation to natural antioxidants from DSS, the concentrations used were satisfactory for maintaining the oxidative stability of the products for three months of frozen storage. These results agree with those reported by others investigators [50] -[52] , who reported that mustard leaf, wine industry residues extract and carnosic acid respectively, controlled the lipid oxidation of raw meat products.
The mechanism of the protective effect may be due to the fact that the DSS is rich in SOD who acts purging the oxidized systems from superoxide radical anion (specific effect) [53] , phenolic compounds that acts as primary antioxidants or free radical terminators [54] , vitamin C (ascorbic acid) whose antioxidant mechanisms are based on hydrogen atom and electron donation to lipid radicals, quenching of singlet oxygen and removal of molecular oxygen [55] . Also, the DSS antioxidant activity has been related with tocopherols and flavonoids regeneration.
Figure 2 shows the inhibition or the reducing percent of lipid oxidation. The samples treated with 1% of dry soybean sprouts present higher reduction of oxidation than others concentrations of the natural antioxidant. The reducing percentage calculated for TBARS reached 70% in the samples with 1% DSS, presenting the same value than those treated with synthetic antioxidant (BHA) at the end of storage, thus indicating high protection of this product against lipid oxidation.
3.4. Microbiological Analysis
The shelf-life of raw meat is usually limited by microbial spoilage; hence the control of microbial contamination is a major concern for the meat industry. The assay was performed at zero day of storage to samples C, EP 0.01% BHA and EP 1% DSS (due to its highest reducing percent of lipid oxidation) and microbial populations were ranged between 4.30 to 4.84 log cfu/g (p < 0.05). These results agree with the informed by others investigators [37] [45] . Addition DSS affect the microbial status of beef patties relative to the controls, presumably due to the product formulations, although did not exceed 6 log cfu/g [37] .
4. Conclusion
Results of the present study indicate that dry soybean sprouts possess a good level of SOD, high total phenolics and flavonoids content, and also of vitamin C. Although reduction power was not good enough, DPPH and ABTS radicals scavenging activity were elevated. When DSS were incorporated to meat samples at different concentrations, not affected nutritional value or lipidic profile of enriched beef patties with soybean oil, and meat color, specifically red, was preserved. The lipid oxidation was inhibited in all cases, and was strongly effective to concentrations of 1% DSS. The results shown in this research suggest that dry soya sprouts could be used as a natural antioxidant for meat and meat products. Further investigations are necessary to evaluate their sensorial influence and microbiological safety during storage.
 (a)
(a) (b)
(b) (c)
(c)
Figure 1. Effect of dry soybean sprouts (0%; 0.5%; 1% and 2%) on (a) Conjugated dienes; (b) Peroxide values; and (c) TBARS in enriched patties with soybean oil. Data represent the means ± SD (p < 0.05).
 (a)
(a)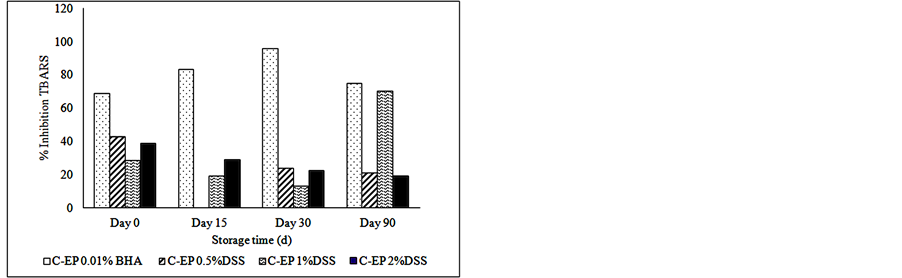 (b)
(b)
Figure 2. Inhibition percent of lipid oxidation by different DSS concentration. (a) Inhibition percent on hydroperoxides formation; and (b) Inhibition percent on TBARS formation.
Acknowledgements
This research was supported by Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET, Argentina), and Universidad Nacional de Chaco Austral. The authors acknowledge the contributions of Cooperativa la Unión, Ltda.