Hydrogen Generation by Reforming of Sodium Hypophosphite on Cobalt-Boron Oxides Containing Catalyst ()
1. Introduction
The rapid depletion of traditional energy sources (crude oil, natural gas, coal, etc.) forces scientists to work on problems of development of alternative energy sources. An unlimited amount of hydrogen is a very attractive candidate as an ecologically friendly source of energy. Hydrogen, used in fuel cells as fuel and obtained by catalytic reforming of natural gas or crude oil, contains carbon monoxide. Even a small amount of CO poisons electrodes containing Pt and Pd and negatively effects performance of fuel cell. Storage and transportation of hydrogen are also an issue. Storage of hydrogen under high pressure, in a liquid form or cryoadsorbed form cannot satisfy demands of safety. The most promising way to store hydrogen is to keep it bound in hydrides of different metals [1] - [9] .
In this work we present the possibility of utilization of sodium hypophosphate (NaH2PO2) as a source of hydrogen for fuel cells. In a process of chemical nickel-plating at temperatures of 80˚C - 95˚C, hydrogen is generated due to interaction of NaH2PO2 with water. This process takes place with high rate only on metals which exhibit catalytic activity at a high temperature. For this purpose, the surface is usually treated with solution of SnCl2 and activated with compounds of Pd2+. Sn2+ ions on the surface reduce Pd2+ ions to metal Pd, thin catalytic layer of which uniformly covers the treated surface. For example, total reaction of reduction of Ni2+ ions reduction on surfaces activated by metal palladium can be presented as [10] - [16] :
 (1)
(1)
Therefore selection of commercially competitive and efficient catalysts, which will be active at low temperature for hydrogen generation from sodium hypo- phosphite, is problem.
The goal of the present work is synthesis of highly active catalyst CoO∙B2O3 for generation of hydrogen from solution of NaH2PO2.
2. Experimental
2.1. Reagents and Methods of Analysis
NaOH, NaH2PO2, CoSO4, NaBH4 grade of “Chemically pure reagents” were used. Solutions were prepared using distilled water.
To synthesize boron containing cobalt catalyst, 20 mL of 0.6 M NaBH4 solution was added dropwise under stirring at temperature of 90˚C (exothermic process) to 20 mL of 1 M CoSO4 solution under stirring (stirring rate 800 rpm) at temperature of 900C (exothermic process). Hot pulp was filtrated and washed with hot water until sulfate ions were removed [2] . The precipitate was dried in the thermostat at 105˚C until the uniform substance was obtained. Content of boron (14.7%) in the mixture was determined by volumetric method and cobalt (44.0%) was determined by spectroscopic method (Perkin Elmer, B3150050). The results showed that we synthesized compound contains CoO∙B2O3 (CoB2O4). The reaction of synthesis can be written as:
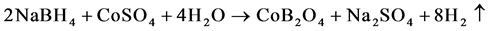 (2)
(2)
Synthesized CoO∙B2O3 was identified by thermographic and X-ray analysis.
Thermogravimetric method of analysis of synthesized CoO∙B2O3 was performed on derivatograph Q-1500 D with rate of heating 10˚C/min. On TG and DTG curves at 350˚C, it can be observed the loss of mass caused by evaporation of adsorbed and bounded water. On DTA curve, in the range of 350˚C - 600˚C, exoeffects with two maximums at temperatures of 420˚C and 500˚C were ob marked. This phenomenon can be explained by overlay of exoeffect of oxidation of CoO by oxygen to Co3O4 on endothermic effect of partial conversion of solid solution (Figure 1).
X-ray investigation of synthesized sample annealed at 350˚C in a muffle furnace for 2 hours was performed on “DRON-3M” diffractometer by CuKα-irradi- ation in monochromatic conditions in the interval of reflection angle 2θ = 20˚ - 65˚. Phase content of studied samples was determined by comparison of experimental set of interplanar distances and relative intensity of diffraction maxima with directory of ASTM. Based on X-ray data the main phase of the sample, annealed at 350˚C is CoO∙B2O3 (2.88 Å, 2.44 Å, 2.08 Å, 2.03 Å, 1.75 Å, 1.65 Å, 1.56 Å, 1.43 Å). In the sample annealed at 600˚C, peaks of new phase of Co3B2O6 were detected together with the main phase peaks of CoO∙B2O3 (3.99 Å, 3.49 Å, 2.69 Å, 2.50 Å, 2.25 Å, 2.09 Å, 1.74 Å, 1.67 Å, 1.53 Å). Identification of Co3O4 (Figure 1, curve DTA, 350˚C - 600˚C), is complicated due to similarity with X-ray picture of CoO∙B2O3.
Surface morphology of synthesized sample (photo 1) was studied by scanning electron microscopy (SEM) with different magnification (×1000, ×10,000 and ×27,000) (Figure 2).
After catalytic decomposition of NaH2PO2 hydrogen was generated in a glass reactor placed in a thermostat and provided with condenser and sealed nozzle
![]()
Figure 1. Derivatogramm of synthesized sample of CoO∙B2O3.
![]()
Figure 2. Surface of synthesized sample of CoO∙B2O3 annealed at 350˚C.
with gas outlet tube connected to the burette (Figure 3). The amount of evolved hydrogen was calculated by the equation:
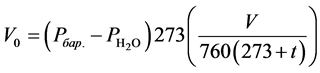 (3)
(3)
V0―volume of hydrogen at normal temperature and pressure (NTP) (ml); V―volume of evolved hydrogen (ml) under experimental conditions;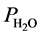 ―pressure of water vapor (mmHg); t―ambient temperature (˚C).
―pressure of water vapor (mmHg); t―ambient temperature (˚C).
Specific rate of hydrogen evolution W (ml∙min−1∙g−1) in the range of temperatures 20˚C - 80˚C is determined by equation:
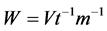 (4)
(4)
V is volume of hydrogen generated at room temperature (ml); t―time of reaction (min); m―mass of catalyst (g).
Based on the method discussed, hydrogen will be obtained on site in fuel cell and problems with storage and transportation of hydrogen will be avoided.
3. Results and Discussions
Hydrolysis of NaH2PO2 is performed in solution of 2 M NaH2PO2 + 2 M NaOH in presence of 0.1 g of CoO∙B2O3 catalyst (dried at temperature 105˚C) until the full evolution of hydrogen.. With increase of temperature of solution, rate of hydrogen evolution is significantly increased: in 60 min at 30˚C, 620 ml of hydrogen was evolved, while at 80˚C the same volume of hydrogen was evolved in 4 min. The rate of generation of hydrogen increased almost 20 times (Figure 4).
Catalytic activity of CoO∙B2O3 was determined in a process of hydrolysis of NaH2PO2 (solution temperature 30˚C) in presence of catalysts annealed at 105˚C, 280˚C, 350˚C and 480˚C. Efficiency of hydrogen evolution reached 95% on a catalyst annealed at 280˚C. Generation of hydrogen on a catalyst annealed at 350˚C was finished in 25 min and the efficiency was 78%. The same time (25
![]()
Figure 3. Scheme of a set up for catalytic decomposition of NaH2PO2.
min) was necessary to generate hydrogen with 74% at a catalyst annealed at 280˚C (Figure 5).
Figure 6 and Figure 7 show results of experiments conducted with catalysts annealed at 280˚C and 350˚C and with rest of conditions as above. The change of temperature of solution from 30˚C to 80˚C accelerated generation of hydrogen about 22 times (from 14 mL/min to 315 mL/min) in the first case (catalyst annealed at 280˚C) and 16 times (from 24 mL/min to 390 mL/min) in the second case (catalyst annealed at 280˚C).
Reactions rate constants, energy of activation and temperature coefficients of reforming rates of sodium hypophosphate reforming rates on CoO・B2O3, catalyst
![]()
Figure 4. Dependence of volume of generated hydrogen during the process of NaH2PO2 hydrolysis with presence of CoO∙B2O3 catalyst at solution temperature (solution volume 20 mL; solution composition―2 M NaH2PO2 + 2 M NaOH; mass of CoO・B2O3 catalyst― 0.1 g): 1―30˚C; 2―40˚C; 3―50˚C; 4―60˚C; 5―80˚C.
![]()
Figure 5. Dependence of volume of generated hydrogen during the process of NaH2PO2 hydrolysis in presence of CoO・B2O3 annealed at different temperatures (solution volume― 20 mL; solution composition 2 M NaH2PO2 + 2 M NaOH; mass of CoO・B2O3 catalyst― 0.1 g; solution temperature 30˚C): 1―480˚C; 2―105˚C; 3―280˚C; 4―350˚C.
![]()
Figure 6. Dependence of volume of generated hydrogen during the process of NaH2PO2 hydrolysis in presence of CoO・B2O3 at solution temperature (solution volume―20 mL; solution composition 2 M NaH2PO2 + 2 M NaOH; mass of CoO・B2O3 catalyst annealed at 280˚C―0.1 g): 1―30˚C; 2―40˚C; 3―50˚C; 4―60˚C; 5―80˚C.
![]()
Figure 7. Dependence of volume of generated hydrogen during the process of NaH2PO2 hydrolysis in presence of CoO・B2O3 at solution temperature (solution volume―20 mL; solution composition 2 M NaH2PO2 + 2 M NaOH; mass of CoO・B2O3 catalyst annealed at 350˚C―0.1 g): 1―30˚C; 2―40˚C; 3―50˚C; 4―60˚C; 5―80˚C.
annealed at 350˚C were determined. Using data of five parallel experiments we built the plot showing dependence of natural log of difference of hydrogen volumes on time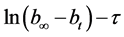 , where
, where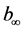 ―hydrogen volume at a normal temperature and pressure (NTP) (ml), allocated until the full decomposition of NaH2PO2,
―hydrogen volume at a normal temperature and pressure (NTP) (ml), allocated until the full decomposition of NaH2PO2,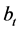 ―hydrogen volume (ml) (NTP), generated in time point τ (Figure 8). Straight lines not crossing the origin of coordinate axes were obtained. Tangent of slop angle of the straight line to an axis of time is equal to a reaction rate constant:
―hydrogen volume (ml) (NTP), generated in time point τ (Figure 8). Straight lines not crossing the origin of coordinate axes were obtained. Tangent of slop angle of the straight line to an axis of time is equal to a reaction rate constant:
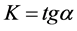 (5)
(5)
![]()
Figure 8. Dependence of logarithm of the difference of the volumes hydrogen on time at different temperatures of solution: 1―30˚C; 2―40˚C; 3―50˚C; 4―60˚C; 5―80˚C (solution volume 20 ml, solution composition 2 M NaH2PO2 + 2 M NaOH; mass of CoO・B2O3 catalyst ―0.1 g).
At each temperature, k30˚ = 8.53 × 10−4 s−1; k40˚ = 1.62 × 10−4 s−1; k50˚ = 3.06 × 10−3 s−1; k60˚ = 5.06 × 10−3 s−1; k80˚ = 1.39 × 10−2 s−1 were calculated.
Using Equations (6) and (7) and rate constants calculated at two temperatures, temperature coefficient and activation energy of chemical reaction were calculated as γ = 0.917; EA = 49.59 kJ∙mol−1.
 (6)
(6)
![]() (7)
(7)
γ―temperature coefficient of rate of chemical reaction; k1 and k2 rate constants at T1 and T2, respectively; R―universal gas constant; EA energy of activation.
4. Conclusions
Catalyst CoO∙B2O3 is synthesized and identified by chemical and X-ray analysis. Thermal stability of the sample is estimated and temperature of conversion is determined.
Synthesized compound CoO∙B2O3 was used as a catalyst during reforming of sodium hypophosphate. Samples annealed at 280˚C - 350˚C demonstrated best catalytic activity. Reforming of NaH2PO2 was conducted in a solution of 2 M (NaH2PO2 + NaOH) at range of temperatures 30˚C - 80˚C; rate constants k30˚ = 8.53 × 10−4 s−1; k40˚ = 1.62 × 10−4 s−1; k50˚ = 3.06 × 10−3 s−1; k60˚ = 5.06 × 10−3 s−1; k80˚ = 1.39 × 10−2 s−1, temperature coefficient γ = 0.917 and activation energy EA = 49.59 kJ∙mol−1 are calculated.
Acknowledgements
The presented project has been fulfilled with the financial support from “Shota Rustaveli National Science Foundation” (Grants № AR/10/3-171/14). Any idea in this publication is possessed by the authors and may not represent the opinion of “Shota Rustaveli National Science Foundation”.