Mathematical Analysis and Simulation of an Age-Structured Model of Two-Patch for Tuberculosis (TB) ()
1. Introduction
Tuberculosis (TB) (short for tubercle bacillus) is a widespread, infectious disease caused by various strains of mycobacteria, usually Mycobacterium tuberculosis (MTB). Tuberculosis typically attacks the lungs, but can also affect other parts of the body [1] . To be infected bacilli must penetrate deep into the alveoli, but the contagiousness of the disease is relatively low and depends on the immune system of subjects. Individuals at highest risk are young children, adults, deficient elderly, and people living in precarious socio-economic conditions, in nursing or whose immunity is deficient (AIDS, immunosuppressive therapy ...) [2] . This is one of the most common old infectious diseases [3] [4] , with about two billion people being currently infected. There are about nine million new cases of infection each year and two million deaths per year according to WHO estimations [3] [5] . For more information, many authors have worked on the epidemiology of tuberculosis [1] - [3] [5] - [13] . In many developing countries in general and sub-Saharan Africa particularly, TB is the leading cause of death, accounting for about two million deaths and a quarter of avoidable adult deaths [11] .
It is well known that factors such as the emergence of drug resistance against tuberculosis, the growth of the incidence of HIV in recent years, as well as other diseases favor the development of Koch bacillus in the body call for improved strategies to control this deadly disease [2] [10] [14] . Last May, the World Health Assembly approved an ambitious strategy for 20 years (2016-2035) to put an end to World TB epidemic (World Day of fight against tuberculosis―March 24, 2015). In literature, several articles discussed about coinfection: TB-HIV/AIDS and the most recent is [2] . Nowadays, it is not a secret for everyone that fighting against infectious diseases is also a fight against poverty. Humans are traditionally organized into well-defined social units, such as families, tribes, villages, cities, countries or regions are good examples of patches [11] [12] . For this study, two subpopulations were considered and each was subjected to a vaccination program. However, only the vaccinated individuals can migrate from one patch to another. Despite that we have neglected the relapse rate, to avoid any risk of treated individuals’ reactivation, any migration between patches was allowed. After proving that the problem is well defined and it has a unique solution if the initial condition is given, we are able to calculate the reproduction of numbers 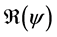 and
and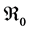 . We have established the existence conditions for three endemic equilibrium points, and the conditions of local and global stability of the equilibrium point without disease. Finally, numerical simulations illustrate clinical outcomes. This paper is organized as follows: Section 2 introduces the two-patch model structured in age to study the dynamics of TB transmission. The existence of positive and unique solutions is demonstrated in Section 3. The point of equilibrium without disease, reproductive numbers
. We have established the existence conditions for three endemic equilibrium points, and the conditions of local and global stability of the equilibrium point without disease. Finally, numerical simulations illustrate clinical outcomes. This paper is organized as follows: Section 2 introduces the two-patch model structured in age to study the dynamics of TB transmission. The existence of positive and unique solutions is demonstrated in Section 3. The point of equilibrium without disease, reproductive numbers 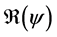 and
and 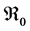 are defined in the section 4 with the local and global stability of the disease-free equilibrium point. The existence of three endemic equilibrium points is proven in Section 5. Some numerical simulation results are given in Section 6. In Section 7, we have a discussion, conclusion and further work.
are defined in the section 4 with the local and global stability of the disease-free equilibrium point. The existence of three endemic equilibrium points is proven in Section 5. Some numerical simulation results are given in Section 6. In Section 7, we have a discussion, conclusion and further work.
2. Parameters and Mathematical Model Formulation
Two-patch age structured model of tuberculosis was considered. The model is to split the population into two subpopulations. The recruitment is only possible in the class of susceptible and the vaccinated individuals were able to migrate between the two subpopulations. Each subpopulation is divided into five classes based on their epidemiological status: susceptible, vaccinated, latent, infectious or treated. We denote these subgroups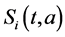 ,
, 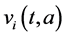 ,
, 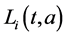 ,
, 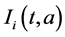 and
and 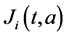 respectively. The birth rate of the patch i is
respectively. The birth rate of the patch i is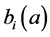 ;
; 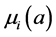 and
and 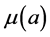 denote the mortality rate related to the disease relative to the patch i and the rate of natural mortality. The time and age depended of the force of infection of the subpopulation i is
denote the mortality rate related to the disease relative to the patch i and the rate of natural mortality. The time and age depended of the force of infection of the subpopulation i is 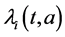 and vaccination rate is
and vaccination rate is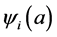 ;
; 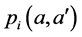 is the probability that an infective individual of age
is the probability that an infective individual of age 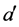 will have contact with and successfully infect a susceptible individual of age a,
will have contact with and successfully infect a susceptible individual of age a, ![]() is the age-specic per-capita contact/activity rate (all of these functions are assumed to be continuous and to be zero beyond some maximum age). A fraction
is the age-specic per-capita contact/activity rate (all of these functions are assumed to be continuous and to be zero beyond some maximum age). A fraction ![]() of newly infected individuals of the sub-population i is assumed to undergo a fast progression directly to the infectious class
of newly infected individuals of the sub-population i is assumed to undergo a fast progression directly to the infectious class![]() . Rates of migration, of susceptible passage to latent infectious state and treatment are respectively
. Rates of migration, of susceptible passage to latent infectious state and treatment are respectively![]() ;
; ![]() and
and![]() . Risk reduction rates of treatment and vaccination are
. Risk reduction rates of treatment and vaccination are ![]() and
and ![]() respectively,
respectively, ![]() ,
, ![]() , in this paper
, in this paper![]() .
.
The age-structured model for the transmission of TB (see Figure 1) is described by the following system of partial differential equations:
![]() (1)
(1)
with initial and boundary conditions:
![]()
and
![]() ,
,
assume that assume that
![]() (2)
(2)
![]()
Figure 1. Flow chart of the two-patch model for tuberculosis disease transmission.
(see Greenhalgh, 1988 [15] and Dietz Schenzle, 1985 [16] ), and
![]() .
.
By summing equations of system (1) and (2), we obtain the following equations for the total population![]() :
:
![]() (3)
(3)
where![]() ;
; ![]() and
and ![]() are respectively the minimum and maximum age of procreation and
are respectively the minimum and maximum age of procreation and ![]() is the maximum age of an individual, with
is the maximum age of an individual, with![]() .
.
Let
![]() (4)
(4)
The system (1) can be normalized as the following system:
![]() (5)
(5)
with boundary conditions
![]()
with![]() . The problem is well-posedness, the methode of proof is the same used in [8] .
. The problem is well-posedness, the methode of proof is the same used in [8] .
3. Existence of Positive Solutions
Consider the Banach space X defined by ![]() endowed with the norm
endowed with the norm
![]() (6)
(6)
where ![]() and
and ![]() is the norm of
is the norm of![]() . Let
. Let
![]() (7)
(7)
The state space of system (5), where![]() , and
, and ![]() denotes the positive cone of
denotes the positive cone of![]() . Let A be a linear operator defined by
. Let A be a linear operator defined by
![]() . (8)
. (8)
To determine the components![]() , we neglect terms of order two and those which are not multiplied by
, we neglect terms of order two and those which are not multiplied by![]() ,
, ![]() ,
, ![]() ,
, ![]() or
or ![]() in system (5) (see [17] ), we obtain:
in system (5) (see [17] ), we obtain:
![]()
After replacing![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() and
and ![]() by
by![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
,![]() ,
, ![]() ,
, ![]() ,
, ![]() ,
, ![]() in the system (a) respectively, the coordinates of
in the system (a) respectively, the coordinates of ![]() are obtained from straight expressions (note that each
are obtained from straight expressions (note that each ![]() with respect to
with respect to ![]() are given by:
are given by:
![]() . (9)
. (9)
With
![]()
where ![]() is the domain given by:
is the domain given by:
![]()
And ![]() denotes the set of absolutely continuous functions on
denotes the set of absolutely continuous functions on![]() . We also define a nonlinear operator
. We also define a nonlinear operator ![]() by:
by:
![]() (10)
(10)
where ![]() is a bounded linear operator on
is a bounded linear operator on ![]() given by
given by
![]() . (11)
. (11)
Let
![]()
![]() (12)
(12)
where
![]()
According to these results we have the following results (see [17] - [19] ):
Lemma 1. The operator F is continuously Fréchet differentiable on X.
Lemma 2. The operator A generates a ![]() -semigroup of the bounded linear operators
-semigroup of the bounded linear operators ![]() and the space
and the space ![]() is positively invariant by
is positively invariant by![]() .
.
Theorem 1. For each ![]() there are a maximal interval of existence
there are a maximal interval of existence ![]() and a unique continuous mild solution
and a unique continuous mild solution![]() ,
, ![]() for (12) such that
for (12) such that
![]()
Proof. The proof of this theorem can be found in [18] - [20] . W
4. The Disease-Free Steady State
4.1. Determination of the Disease-Free Equilibrium
A steady state ![]() of system (5) must satisfy the following time-independent system of ordinary differential equations:
of system (5) must satisfy the following time-independent system of ordinary differential equations:
![]() (13)
(13)
with initial value conditions
![]()
Therefore, we obtain the disease-free steady state
![]() . (14)
. (14)
4.2. Calculation of the Reproduction Numbers ![]() -
-![]()
To study the stability of the disease-free steady state, we denote the perturbations of system by
![]() . (15)
. (15)
The perturbations satisfy the following equations:
![]() (16)
(16)
with boundary conditions:
![]()
we consider the exponential solutions of system (16) of the form:
![]() . (17)
. (17)
The system (16) becomes:
![]() (18)
(18)
with boundary conditions:
![]()
Let
![]() . (19)
. (19)
From Equation (18), we obtain:
![]() (20)
(20)
![]() . (21)
. (21)
Hence, by Equations ((20) and (21)) after changing order of integration, we obtain:
![]() . (22)
. (22)
Injecting (22) in the expression of![]() , and dividing both sides the expression by
, and dividing both sides the expression by ![]() (since
(since![]() ), we get the characteristic equation:
), we get the characteristic equation:
![]() . (23)
. (23)
Denote the right-hand side of Equation (23) by ![]() i.e.:
i.e.:
![]() . (24)
. (24)
We define the net reproductive number as![]() , i.e.
, i.e.
![]() . (25)
. (25)
We can obtain an expression for ![]() in a similar way as the derivation of
in a similar way as the derivation of ![]() by considering Equation (1) without vaccination; i.e., by assuming that
by considering Equation (1) without vaccination; i.e., by assuming that ![]() and neglecting the equation of vaccinated. It can be shown that
and neglecting the equation of vaccinated. It can be shown that ![]() which is called the basic reproductive number (when a purely susceptible population is considered) (see [8] ).
which is called the basic reproductive number (when a purely susceptible population is considered) (see [8] ).
![]() (26)
(26)
Let
![]()
4.3. Local Stability of the Disease-Free Equilibrium
Theorem 2. The infection-free steady-state (5) is locally asymptotically stable (l.a.s.) if ![]() and unstable if
and unstable if![]() .
.
Proof. Noticing that
![]()
We know that Equation (23) has a unique negative real solution ![]() if, and only if,
if, and only if, ![]() , hence,
, hence, ![]() (Also, Equation (23) has a unique positive (zero) real solution if
(Also, Equation (23) has a unique positive (zero) real solution if ![]() (
(![]() ). To show that
). To show that ![]() is the dominant real part of roots of
is the dominant real part of roots of![]() , we let
, we let ![]() be an arbitrary complex solution to Equation (23). Note that
be an arbitrary complex solution to Equation (23). Note that
![]()
indicating that![]() . It follows that the infection-free steady state is l.a.s. if
. It follows that the infection-free steady state is l.a.s. if![]() , and unstable if
, and unstable if![]() . W
. W
In this corollary, we have the three cases of the unstability of the disease free equilibrium.
Corollary 1. 1) whenever ![]() and
and![]() , the disease free is locally asymptotically stable in the first patch and unstable in the second.
, the disease free is locally asymptotically stable in the first patch and unstable in the second.
2) whenever ![]() and
and![]() , the disease free is unstable in the first patch and locally asymptotically stable in the second.
, the disease free is unstable in the first patch and locally asymptotically stable in the second.
3) whenever ![]() and
and![]() , the disease free is unstable in the two patches.
, the disease free is unstable in the two patches.
4.4. Global Stability of the Disease-Free Equilibrium
Since ![]() and
and ![]() are bounded, there exists a positive constant
are bounded, there exists a positive constant ![]() that satisfies
that satisfies
![]() (*).
(*).
Corollary 2. Assume that![]() , then we have
, then we have
![]()
Theorem 3. The disease-free equilibrium of system (5) is globally asymptotically stable if ![]() and
and![]() .
.
Proof. The proof consist to show that
![]() ;
;![]() ;
;![]() ;
;
![]() and
and![]() , when
, when ![]()
Integrating system (5) along characteristic lines we get
![]() (27)
(27)
![]() . (28)
. (28)
Injecting (27) in (28), and changing order of integration, we obtain:
![]() . (29)
. (29)
Injecting (29) in![]() , and changing order of integration, we obtain:
, and changing order of integration, we obtain:
![]() . (30)
. (30)
By using corollary 2, inequality (*) and Fatou’s lemma, we have
![]()
Since![]() ,
, ![]()
![]()
![]() W
W
Corollary 3. The disease-free equilibrium is globally asymptotically in:
1) the first sub-population if ![]() and
and ![]()
2) the second sub-population if ![]() and
and ![]()
For this disease can disappear without any form of intervention, according to these results we must ensure that there is no new infected and the infectious rate does not reach a certain spread.
5. Existence of an Endemic State
There exists three endemic steady state of system (5) whenever![]() .
.
5.1. The First Boundary Endemic Equilibrium
Theorem 4. A boundary endemic equilibrium of the form ![]() whenever
whenever ![]() and
and![]() . This means that the disease is endemic in the first sub-population and dies out in the second sub-population.
. This means that the disease is endemic in the first sub-population and dies out in the second sub-population.
Proof. The method commonly used to find an endemic steady state for age-structure models consists of obtaining explicit expressions for a time independent solution of system (5)
![]() satisfies the following equations:
satisfies the following equations:
![]() (31)
(31)
with the initial conditions:
![]()
Let
![]() . (32)
. (32)
Integrating system (31), we obtain:
![]() (33)
(33)
![]() (34)
(34)
![]() (35)
(35)
![]() (36)
(36)
![]() (37)
(37)
![]() (38)
(38)
![]() . (39)
. (39)
By injecting (37) in (34), we obtain:
![]() . (40)
. (40)
Injecting (40) in the expression of![]() , and dividing by
, and dividing by ![]() (since
(since![]() )we obtain:
)we obtain:
![]() . (41)
. (41)
Let![]() , the function define by:
, the function define by:
![]() . (42)
. (42)
Since ![]() i.e. when
i.e. when![]() , so the net reproductive number is given by
, so the net reproductive number is given by
![]()
i.e.
![]()
We now see that an endemic steady state exists if Equation (41) has a positive solution.
Since![]() , hence
, hence![]() . We know that
. We know that ![]() . Hence
. Hence
![]() . (43)
. (43)
Since![]() , from (42) and (43) we obtain:
, from (42) and (43) we obtain:
![]()
In particular, for![]() , we have
, we have![]() , but
, but![]() . Since
. Since ![]() is continous function of
is continous function of![]() , we conclude that
, we conclude that![]() , has a positive solution
, has a positive solution ![]() on
on![]() . This solution may not be unique since H may not be monotone (
. This solution may not be unique since H may not be monotone (![]() depends on
depends on ![]() which is defined implicitly). It follows that when
which is defined implicitly). It follows that when![]() , there exists an endemic steady state distribution which is given by the unique solution of Equation (41) corresponding to
, there exists an endemic steady state distribution which is given by the unique solution of Equation (41) corresponding to![]() . W
. W
5.2. The Second Boundary Endemic Equilibrium
Theorem 5. A boundary endemic equilibrium of the form ![]() whenever
whenever ![]() and
and![]() . This means that the disease is dies out in the first sub- population and is endemic in the second sub-population.
. This means that the disease is dies out in the first sub- population and is endemic in the second sub-population.
Proof. (Ideas of proof) ![]() satisfies the following equations:
satisfies the following equations:
![]() (44)
(44)
with the initial conditions:
![]() . (45)
. (45)
Let
![]() . (46)
. (46)
Integrating system (51), we obtain:
![]() (47)
(47)
![]() (48)
(48)
![]() (49)
(49)
![]() (50)
(50)
![]() (51)
(51)
![]() (52)
(52)
![]() . (53)
. (53)
Hence, by the similar method using in theorem 4, we obtain the result. W
5.3. The Interior Endemic Equilibrium
Theorem 6. An interior endemic equilibrium of the form
![]()
whenever ![]() and
and![]() , which corresponds to case when the disease persists in the two sub-populations.
, which corresponds to case when the disease persists in the two sub-populations.
Proof. ![]() satisfies the following equations:
satisfies the following equations:
![]() (54)
(54)
with the initial conditions:
![]() (55)
(55)
![]() (56)
(56)
Let
![]() (57)
(57)
![]() (58)
(58)
![]() (59)
(59)
![]() (60)
(60)
![]() (61)
(61)
![]() (62)
(62)
By injecting (58) in (59), we obtain:
![]() (63)
(63)
By injecting (63) in the expression of![]() , and dividing by
, and dividing by ![]() (since
(since![]() ) we obtain:
) we obtain:
![]() (64)
(64)
Let![]() , the function define by:
, the function define by:
![]() (65)
(65)
Since ![]() i.e. when
i.e. when![]() , so the net reproductive number is given by
, so the net reproductive number is given by
![]() , i.e.
, i.e.
![]()
We now see that an endemic steady state exists if Equation (64) has a positive solution. Since
![]() , hence
, hence![]() . We know that
. We know that ![]() . Hence
. Hence
![]() (66)
(66)
Since![]() , from (65) and (66) we obtain:
, from (65) and (66) we obtain:
![]()
In particular, for![]() , we have
, we have![]() , but
, but![]() . Since
. Since ![]() is continous function of
is continous function of![]() , we conclude that
, we conclude that![]() , has a positive solution
, has a positive solution ![]() on
on![]() . This solution may not be unique since
. This solution may not be unique since ![]() may not be monotone (
may not be monotone (![]() depends on
depends on ![]() which is defined implicitly). It follows that when
which is defined implicitly). It follows that when![]() , there exists an endemic steady state distribution which is given by the unique solution of Equation (64) corresponding to
, there exists an endemic steady state distribution which is given by the unique solution of Equation (64) corresponding to![]() . W
. W
5.4. Simulation
In this section, when ![]() and
and ![]() we will evaluate the impact of BCG vaccine and the birth rate of the population in the dynamics of spread of TB. Assuming that all parameters are the same in both patches except the vaccine rate, we observe an increase in the number of infected if the vaccination rate decreases (Figure 2). Also taking the same parameters except birth rates, we see an increased number of infected if the rate increases (Figure 3).
we will evaluate the impact of BCG vaccine and the birth rate of the population in the dynamics of spread of TB. Assuming that all parameters are the same in both patches except the vaccine rate, we observe an increase in the number of infected if the vaccination rate decreases (Figure 2). Also taking the same parameters except birth rates, we see an increased number of infected if the rate increases (Figure 3).
![]()
Figure 2. Evolution of the number of latents individuals with ![]() and
and![]() .
.
![]()
Figure 3. Evolution of the number of latents individuals with ![]() and
and![]() .
.
When ![]() and
and ![]() (
(![]() and
and![]() ), we have the evolution of the number of infectious individuals (Figure 4).
), we have the evolution of the number of infectious individuals (Figure 4).
6. Discussion, Conclusion and Future Work
In this paper, an age structured model of two-patch for tuberculosis was analyzed and discussed. Each sub-population is subjected to a vaccination program. Apart from age; the vaccinated compartment, we introduced as a class of treated in the model proposed by Tewa J. Jules in [11] and allowed the migration of vaccinated population. The same result was found if the most susceptible migrated too. Although some studies have shown an ineffectiveness of BCG in the prevention of tuberculosis [21] , our work demonstrated the contribution of BCG in the process of eradicating TB. The negative impact of the increase in the birth rate was shown. If we neglect the mortality death rate linked to the disease, we obtain the only usual condition of global stability to the disease free equilibrium i.e.![]() . It remains for us many challenges such as the endemic equilibrium points of this model and the one of [8] to deal with. For future work, in order to study the real impact of the tuberculosis migration in the dynamic of the expansion of the disease, we will use this model and authorize the migration of all individuals (i.e. susceptible, infected, infectious, vaccinated and treated).
. It remains for us many challenges such as the endemic equilibrium points of this model and the one of [8] to deal with. For future work, in order to study the real impact of the tuberculosis migration in the dynamic of the expansion of the disease, we will use this model and authorize the migration of all individuals (i.e. susceptible, infected, infectious, vaccinated and treated).
Acknowledgements
We thank the Editor and the referee for their comments. We would like to thank Numerical Analysis student group for their valuable comments and the authors whose works have been used in this article. We also thank the ministry of Higher Education of Research an Innovation who kindly supported the costs of the publication.