Phenolic Contents and Antioxidant Activities of Green Tea with and without Lemon ()
Received 3 March 2016; accepted 14 June 2016; published 17 June 2016

1. Introduction
Tea is one of the most widely used beverages in the world and can be grouped into green, black, white, yellow, oolong and mate. It contains polyphenols, amino acids, proteins, carbohydrates, minerals, caffeine and other volatile compounds. Among the different types of tea, green tea (Camellia sinesis) is the most preferred type as it has many benefits for human health [1] - [3] . Polyphenol, the main component of tea, consists of various types of catechin (C) [3] , in particular, epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate (ECG) and epigallocatechin-3-gallate (EGCG), which is the most active catechin in green tea [3] - [5] . Catechin compounds in fresh green tea leaves have been reported to have strong antioxidant activity in comparison with oolong and black tea leaves [4] . This is because green tea leaves are not fermented and catechin oxidation by polyphenol oxidase is prevented, while the oolong tea and the black tea leaves are either fermented to a limited extent or fully fermented, respectively [3] [4] .
Lemon fruits (Citrus lemon) contain several kinds of flavonoids―a group of polyphenolic compound―such as flavone glycoside, flavanone glycoside and polymethoxyflavone [6] [7] . Moreover, it is an excellent source of vitamin C, an antioxidant that improves immune system and prevents oxidative stress related diseases [8] [9] .
There are two methods to determine antioxidant capacity: the electron transfer and the hydrogen atom transfer. Folin-Ciocalteu, DPPH, hydrogen peroxide and reducing power are classified as electron transfer methods [10] . In this study, we aimed to compare the antioxidant activity of polyphenols in green tea and green tea supplemented with lemon using different spectrophotometric assays, to estimate the best type of tea that provides a better antioxidant effect. As far as we are aware, there are no studies that have been done on local Saudi green tea brand supplemented with lemon, to evaluate the total phenolic content and the antioxidant activity using five different mechanisms.
2. Materials and Methods
2.1. Materials
Classic green tea and green tea supplemented with lemon were obtained from Local Saudi Market, Folin-Cio- calteu’s phenol reagent, anhydrous sodium carbonate, 2,2-Diphenyl-1-picrylhydrazyl (DPPH), ethanol, hydrogen peroxide, phosphate buffer saline (PBS), ascorbic acid, potassium ferricyanide, trichloroacetic acid (TCA), ferric chloride, ferrous chloride, Ferrozin and ethylenediaminetetraacetic acid (EDTA) were purchased from Sigma-Aldrich Chemical Co. (Pool, UK).
2.2. Sample Preparation
The experimental tea samples were prepared as traditionally consumed by Saudi people. A 10 mg/ml of each sample was prepared by adding hot boiling water to tea samples. The mixture was left for 10 minutes at room temperature, after that the antioxidant mechanisms were evaluated. Three different concentrations (2.5, 5 and 10 mg/ml) were prepared, to estimate the ability of different concentrations as reducing agents.
2.3. Determination of Polyphenol by Follin Reagent
The amount of total phenolic content was quantified according to Folin-Ciocalteu method [11] . First, 0.5 ml of each sample was transferred into test tubes and 5 ml of deionized water was added to each sample. Then 0.5 ml of Folin’s reagent was added. The mixtures were left for 5 minutes at room temperature. After that, 1 ml of 2% sodium carbonate solution was added to the mixtures and incubated for 1 hour in dark room. The color formed was measured at 750 nm.
2.4. DPPH Radical Scavenging Activity
DPPH radical scavenging activity was measured by [12] method. First, 250 µl of each sample was added to 99.5% ethanol. Then, 62.5 µl of DPPH was added to the previous mixture and then incubated for 1 hour in dark place. The absorbance was measured at 517 nm. Radical scavenging activity was expressed as inhibition percentage and was calculated using the following formula:
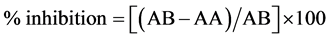
where (AB) = absorption of blank sample and (AA) = absorption of sample.
2.5. Hydrogen Peroxide Radical Scavenging Activity
Scavenging activity of hydrogen peroxide was measured according to [13] . First, 1 ml of the sample was added to 0.6 ml of 40 mM hydrogen peroxide. The absorbance value of the reaction mixture was recorded at 230 nm. Radical scavenging activity was expressed as inhibition percentage and was calculated by the following equation:

where (AB) = absorption of blank sample and (AA) = absorption of sample.
2.6. Reducing Power
The reducing power assay was performed as described by [14] . First, 2.5 ml of 0.2 M phosphate buffer pH 6.6 and 2.5 ml of 1% potassium ferric cyanide were added to 1 ml of the sample and mixed well then incubated for 30 minutes at 50˚C. Then, 2.5 ml of 10% Trichromoacetic acid was added to the previous mixture and centrifuged at 4˚C for 10 minutes at 1600 rpm. After that, 2.5 ml of the supernatant was mixed with 2.5 ml deionized water and 0.5 ml of 1% ferric chloride. The absorbance was measured at 700 nm.
2.7. Iron Chelating Activity
The iron chelating activity was determined according to the procedure mentioned by [15] . A sample 500 µl was added to 50 µl of 2 mM ferrous chloride and 1.5 ml distilled, and then the mixture was vortexed for 30. A 100 µl of 5 mM Ferrozin was added to the previous mixture and was incubated for 10 minutes at room temperature. The absorbance was measured at 562 nm. Ferrous ion chelating capacity was calculated using the following formula:
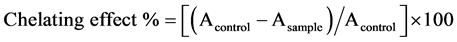
2.8. Statistical Analysis
The data were statistically analyzed using GraphPad Prism 6. Results with p < 0.05 were considered statistically significant. All experiments were performed in triplicate and the values were expressed as mean ± SD. The differences between the samples were assessed using unpaired t-test.
3. Results
3.1. Total Polyphenol Content
Total polyphenol content was estimated by Folin-Ciocalteu’s method. Gallic acid was used as a standard. The content of polyphenols for green tea and green tea supplemented with lemon were 678.7 and 957.3 µg of Gallic acid/10mg of tea, respectively showing that green tea supplemented with lemon has higher polyphenol contents than green tea.
3.2. DPPH Radical Scavenging Activity
Scavenging of DPPH is done by hydrogen/electron Transfer from a given antioxidant to DPPH [16] . The method is based on the reduction of alcoholic solution of the stable free radical DPPH by providing hydrogen atoms from antioxidant molecules to convert DPPH from radical form to non-radical form DPPH-H [17] . The percentages of inhibition caused by green tea and green tea supplement with lemon were 91% and 99% respectively. In this experiment, a significant reduction in the absorbance of DPPH radical was observed. Figure 1 shows that green tea with lemon has more effect in reducing DPPH radicals than green tea at the same concentration (10 mg∙ml−1).
3.3. Hydrogen Peroxide Radical Scavenging Activity
The ability of green tea and lemon to scavenge H2O2 was measured at 320 nm. Since the samples contain phenolic compounds, H2O2 can be converted into H2O by donating electrons [17] . At 10 mg∙ml−1 concentration, green tea with lemon showed a significant H2O2 scavenging activity compared with green tea alone as shown in Figure 2. The percent of inhibition activity of H2O2 of green tea and green tea with lemon were 67% and 93%, respectively.
![]()
Figure 1. Radical scavenging activity of green tea without and with lemon. The value expressed as mean ± SD (n = 3) of triplicate measurements. Comparisons of means were ma- de using unpaired t-test (*p < 0.05).
![]()
Figure 2. H2O2 scavenging activity of green tea without and with lemon. The value expressed as mean ± SD (n = 3) of triplicate measurements. Comparisons of means were made using unpaired t-test (**p < 0.005).
3.4. Reducing Power
In this experiment, the antioxidant compounds convert the oxidation form of iron (Fe+3) in ferric chloride to ferrous (Fe+2). The reducing powers of green tea and green tea supplemented with lemon were presented in Figure 3, and it is clearly shown that, with increased concentrations, the reducing power will also be increased. At all the tested concentrations, the reducing activity of green tea supplemented with lemon was significantly higher than that of green tea. The p values were 0.03, 0.0003 and 0.03 for 2.5, 5 and 10 mg∙ml−1 respectively.
![]()
Figure 3. Reducing power of different concentrations of green tea without and with lemon. The value expressed as mean ± SD (n = 3) of triplicate measurements. Comparisons of means were made using unpaired t-test (*p < 0.05 and ***p < 0.005).
3.5. Iron Chelating Activity
One of the mechanisms of antioxidant action is the chelation of transition metals that prohibiting stimulation of hydrogen peroxide dissociation [18] . This method is based on the inhibition of ferrous-ferrozin complex formation by the antioxidant compound present in the green tea and lemon. By comparing the two results, it can be seen that there is no significant difference in iron chelating activity at the same concentration (10 mg∙ml−1) in both samples (Figure 4).
The standard metal chelator agent used in this experiment was EDTA. Ferrous chelating activity of EDTA was 97% while for green tea and green tea with lemon, chelating activity were lower 83% and 84%, respectively (Figure 5).
4. Discussion
Green, black and oolong tea are three different types of tea that different from each other by fermentation processing [4] . Black tea and oolong tea are fully fermented or semi fermented, respectively during fermentation process [4] [19] . However, green tea is not fermented. It is processed by preventing oxidation of catechin [19] . As a result, the flavanol group of polyphenols called catechins, the major bioactive compounds of green tea, are found in higher concentrations compared to black and oolong tea [4] [20] - [22] . Therefore, the order of antioxidant activity is: green tea > oolong tea > black tea [19] [21] . Based on that, green tea has powerful antioxidantactivity which is responsible for important biological activity and have many health advantages [22] [23] . In general, many studies, reported the efficiency of green tea to scavenge free radicals. One study [24] reported that green tea has high radical scavenging ability by using DPPH free radical scavenging method. Previous studies showed that many bioactive compounds have been found in lemon such as phenolic acid, ascorbic acid, citric acid, limonoids, carotenoids, minerals and flavanone (hesperidin and naringin) [25] [26] . The benefits of polyphenols came from their high antioxidant capacity which prevents the development of many diseases associated with oxidative stress [27] . In our results, green tea with lemon showed more polyphenol contents than green tea and this might be due to the presence of additional phenolic compounds in lemon that increase the amount of antioxidantsin our body.
Flavonoids have a direct role in scavenging free radicals produced mostly as reactive oxygen species (ROS). [28] found that naringin, which is a part of lemon flavanone, can stimulate the immune system defenses to avoid oxidative damage caused by oxidation, by increasing the activity of the body’s antioxidant enzymes including catalase, glutathione peroxidase and superoxide dismutase. These enzymes catalyze the dismutation of superoxide radical, which is the most common free radical in the body, into hydrogen peroxide (H2O2). As a result, the addition of lemon might increase the ability of samples to reduce the amount of H2O2 [20] . A study performed by [29] showed that there is a relationship between flavonoid structure and their radical scavenging. The powerful
![]()
Figure 4. Ferrous chelating activity of green tea without and with lemon. The value expressed as mean ± SD (n = 3) of triplicate measurements. Compression of means was made using unpaired t-test.
![]()
Figure 5. Ferrous chelating activity of green tea without and with lemon compared with EDTA at 0.1 mg∙ml−1. The value expressed as mean ± SD (n = 3) of triplicate measurements. Comparisons of means were made using a one-way ANOVA followed by Bonferroni’s test (***p < 0.0005).
radical scavenging of lemon flavonoids is due to the presence of the ortho-dihydroxy structure in either ring A or ring B, the hydroxyl moiety on position 3 in combination with the oxo group at position 4 and the presence of a C2-C3 double bond in ring C as shown in Figure 6.
For that, this flavonoid has the ability to scavenge DPPH free radicals by rapidly donating hydrogen atom from hydroxyl group to radicals and it is visually noticeable by the change in the color from purple to yellow as shown in Figure 7.
Furthermore, several studies reported that lemon has other important natural chemical components including limonoids, carotenoids, phenolic acid and pectin that also have different levels of free radical scavenging. These components can neutralize free radicals and therefore prevent some of the damages caused by free radicals
![]()
Figure 6. Structural features of flavonoid with a high radical scavenging activity [29] .
![]()
Figure 7. Scavenging of DPPH free radical by a flavonoid [29] .
[30] - [32] . From all these studies, we noticed that there is a relationship between the scavenging activity and the flavonoid content of lemon [28] . This explains to some degree why green tea supplemented with lemon has more ability to scavenge free radical than green tea alone. Reducing power of bioactive compounds may serve as a significant reflection of its potential antioxidant activity [8] . Compounds with reducing power can act as electron givers and can react with free radicals to become steady and prevent radical chain reaction [33] . Therefore, the reducing activity of green tea supplemented with lemon was significantly higher than that of green tea.
Our results in iron chelating activity assay showed that the ability of lemon to chelate iron was weak. In agreement with our results, the study by [34] , indicated that naringin did not chelate iron completely because it was incapable to overrun all active sites of iron. Moreover, a study by [35] indicated that citric acid, which is the main component of lemon, was not a good chelating agent for ferrous ion and its chelating ability was low. The high chelating iron activity in our results derived from green tea might be due to the specific functional group in its catechin flavanols structure [35] .
5. Conclusion
In conclusion, our results indicated that green tea supplemented with lemon has a better antioxidant activity compared to green tea without lemon supplementation, due to the presence of additional phenolic compound in lemon. Further researches are needed using different tea brands with different supplements, in order to estimate the best type of tea supplementation that provides the optimal antioxidants for potential health benefits.
Acknowledgements
The research team would like to thank Science Research & Innovation Unit at the Faculty of Science, King Abdulaziz University for supporting this work
NOTES

*Corresponding author.