Received 17 March 2016; accepted 22 May 2016; published 25 May 2016

1. Introduction
The emission of greenhouse gases, which warms the Earth’s surface and atmosphere, is an urgent global problem. Room temperature ionic liquids (RTILs) have attracted considerable attention for the capture of carbon dioxide (CO2) in efforts to counteract global warming. The capture of CO2 using an RTIL was first reported by physically dissolving supercritical CO2 (scCO2) at high temperature and pressure into 1-butyl-3-methylimidazo- lium hexafluorophosphate, [C4mim][PF6] [1] , and the pressure-CO2 molar fraction phase diagram was constructed at 40˚C. Since then, various theoretical [2] - [6] and experimental [7] - [13] investigations have been conducted to further develop techniques for CO2 capture and storage in green chemistry. Systematic studies reveal that scCO2 is highly soluble in the bis(trifluoromethanesulfonyl)imide (TFSI−) anion-based RTIL [10] and the polymerization of RTILs has been shown to allow the reversible and fast sorption and desorption of normal CO2 (nCO2) [8] . To apply RTILs in actual industrial applications, developing a cost-effective system that does not require high temperature or pressure for nCO2 capture remains necessary.
As additive effect, isomer effects of alcohols in the RTILs were observed distinctly [14] - [17] . A previous study estimated the molecular interactions between RTILs and propanol on desorption time measured under vacuum [14] . The results indicated that 1-propanol interacts more strongly with RTILs than does 2-propanol. In addition, Raman spectroscopy revealed that the propanol isomer effect is related to the conformations of TFSI− anion, which can exist as two stable conformers, cis (C1) and trans (C2) [18] [19] . C1 and C2 conformers of TFSI− originate from the competition between the alkyl side-chain length of the Cnmim+ cation and the propanol isomer effect. Recently, butanol isomer effect was reported in [Cnmim][TFSI]-based systems with four types of butanol [17] , owing to the different hydrophobicities of four types of butanol. The upper critical solution temperatures (UCSTs) in the phase diagrams were clearly separated with increasing alkyl side-chain length of the Cnmim+ cation.
Systematic CO2 solubility experiments have demonstrated that TFSI− exhibits good CO2 capture ability in pure RTILs systems [20] . Isomer effects have been observed in TFSI−-based RTILs, including [Cnmim][TFSI] (2 ≤ n ≤ 10) [15] . The established binary phase diagrams and UNIQUAC (universal quasichemical) interaction parameters indicate that the interactions of 1-propanol with RTILs differ significantly from the interactions between 2-propanol and RTILs.
In this study, we optimized physical sorption of nCO2 at room temperature and ambient pressure. The dilution of RTILs with 2-propanol promoted nCO2 capture, and stabilized the liquid mixing state. The amount of captured nCO2 was related to the torsion angle of the TFSI− anion, which was calculated by density functional theory (DFT). The propanol isomer effect and torsion angle of TFSI− anion had critical effects on the level of nCO2 absorption in the propanol-rich region, which is desirable for decreasing the cost of carbon capture operation.
2. Materials and Methods
Hydrophobic RTILs were used in this study to prevent contamination by water from atmospheric moisture. TFSI− is commonly used as the anion in hydrophobic RTILs. In this study, we tested four quaternary ammonium cations, i.e., N,N-diethyl-N-methyl-N-(2-methoxyethyl)ammonium (DEME+), ethyldimethylpropylammonium, (N1123+), N,N,N-trimethyl-N-propyl ammonium (N3111+), N-trimethyl-N-butylammonium (N4111+), N-tributyl-N-me- thylammonium (N4441+) and methyltrioctylammonium (N8881+), two quaternary phosphonium cations, i.e., triethylpentylphosphonium (P2225+) and tributyl methyl phosphonium (P4441+), and one prototype cation, i.e., 1-butyl-3-methylimidazolium (C4mim+). All RTILs were obtained from IoLiTecCo.1-Propanol (99.5%) and 2- propanol (99.5%; Kanto Chemical Co.) were used as additives.
For CO2 sorption, a CO2 flowing system was assembled. A schematic of the CO2 sorption system is illustrated in Figure 1. Mixtures were put into a glass-type sample bottle (30 cc).
The sample bottle was placed on a container with flowing gas. The sample container was immersed in an ethanol bath (Yamato Scientific Co., BB301) with flowing CO2 gas (30 mL/min) for 10 min. Temperature stability was within 0.1˚C (15˚C ≤ T ≤ 30˚C). Within 5 s, the sample was moved to the electric balance (HR-202i, A & D Co.), which monitored the desorption process of nCO2. Gas selectivity testing was conducted using O2 and N2 gases.
To determine the phase diagrams of the RTIL-propanol mixtures, samples were cooled from 30˚C to −50˚C using an ethanol bath (Yamato Scientific Co., BE200). By visual cloud-point determinations, accuracy of the clouding temperatures was found to be within 0.5˚C. A liquid N2 pot was used as a supplement for further cooling. The minimum temperature (−50˚C) is limited by viscous ethanol at low temperature. The temperature was monitored by a Pt100 temperature sensor (Netsushin Co.). The cooling rate was 1.5 C/min.
The conformational stabilities of the mixtures were examined by Raman spectroscopy using a micro-Raman spectrometer (RA-07F, Seishin-Shoji) in backscattering mode equipped with a monochromator (500M, Horiba JobinYvon) and a charge-coupled device detector (Symphony, Horiba JobinYvon). Radiation at 532 nm from a
![]()
Figure 1. Schematic illustration of nCO2 sorption assembly.
Nd:YAG laser (power = 50 mW) was used as the excitation source.
3. Results and Discussion
3.1. nCO2 Capture in RTIL-Propanol Mixtures
Figure 2 shows the amount of captured nCO2 as a function of propanol concentration in the [N4111][TFSI]- propanol system at a temperature of 25˚C. The molar fraction of nCO2 was calculated as,
 (1)
(1)
where nIL and 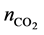 are the moles of RTILs and nCO2, respectively. We did not consider the amount of propanol in this study, as propanol is relatively inexpensive compared with the RTILs. The results show that the addition of 2-propanol can promote nCO2 capture in the propanol-rich region. In contrast, 1-propanol did not enhance nCO2 capture. The value of η for the 1-propanol-based mixtures remained almost constant with changing propanol concentration; this is a typical isomer effect of propanol, as indicated by the phase diagrams [15] . The isomer effect of nCO2 capture is discussed in the next section along with liquid stability. The above tendency is also seen in other systems. The molar fractions at the points of maximum nCO2 sorption for all RTILs-propanol systems studied herein are indicated in Figure 3. Here, temperature was fixed at 25˚C. The nCO2 sorption of the 1-propanol-based mixture was larger than that of the 2-propanol one only for the [DEME][TFSI] system. Relative large values of η were obtained in the quaternary ammonium cation-based systems. In contrast, the phosphonium and imidazolium systems exhibited lower nCO2 capture abilities. The high efficiency of nCO2 capture in the quaternary ammonium cation-based systems can be attributed to the syntheses of these cations. An example of synthesis using the Halogen-free carbonate ester method [21] can be written as follows:
are the moles of RTILs and nCO2, respectively. We did not consider the amount of propanol in this study, as propanol is relatively inexpensive compared with the RTILs. The results show that the addition of 2-propanol can promote nCO2 capture in the propanol-rich region. In contrast, 1-propanol did not enhance nCO2 capture. The value of η for the 1-propanol-based mixtures remained almost constant with changing propanol concentration; this is a typical isomer effect of propanol, as indicated by the phase diagrams [15] . The isomer effect of nCO2 capture is discussed in the next section along with liquid stability. The above tendency is also seen in other systems. The molar fractions at the points of maximum nCO2 sorption for all RTILs-propanol systems studied herein are indicated in Figure 3. Here, temperature was fixed at 25˚C. The nCO2 sorption of the 1-propanol-based mixture was larger than that of the 2-propanol one only for the [DEME][TFSI] system. Relative large values of η were obtained in the quaternary ammonium cation-based systems. In contrast, the phosphonium and imidazolium systems exhibited lower nCO2 capture abilities. The high efficiency of nCO2 capture in the quaternary ammonium cation-based systems can be attributed to the syntheses of these cations. An example of synthesis using the Halogen-free carbonate ester method [21] can be written as follows:
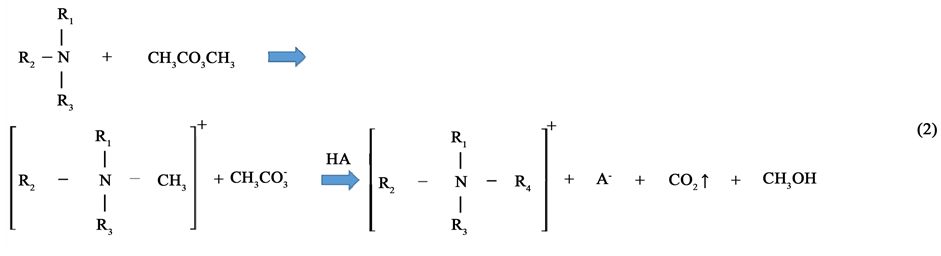
The scheme in Equation (2) leads to the coexistence of quaternary ammonium cation, alcohol and CO2, and provides a clue to explain the high nCO2 capture obtained using quaternary ammonium cations. We predict that the cation, CO2 and alcohol are affirmative each other. Among the RTILs used in this study, the [N4111][TFSI]- 2-propanol system provided the best nCO2 storage.
3.2. Thermal Properties of nCO2 Capture
To investigate the thermal characteristics of nCO2 capture, the η value of the [N4111][TFSI]-80 mol% 2-propanol
![]()
Figure 2. Dependence of nCO2 molar fraction, η (%), on propanol concentration, showing the propanol isomer effect at 25˚C. High nCO2 sorption is observed in the propanol-rich region.
![]()
Figure 3. Maximum nCO2 sorption in various RTILs-propanol systems. With the exception of the [DEME][TFSI]-propanol system, the 2-propanol-based mixtures have a preference for nCO2 capture. Quaternary ammonium RTILs possess relative high abilities for nCO2 capture.
system is plotted as a function of temperature in Figure 4(a). Upon cooling down to 15˚C, almost monotonic increase of η was observed. According to conventional thermodynamics, η decreases with increasing temperature. At lower temperature, CO2 absorption efficiency was elevated. In [N4111][TFSI]-propanol system, however, there is a problem of phase separation in the propanol-rich region. Figure 4(b) reveals the phase diagram of [N4111][TFSI]-1-propanol and -2-propanol. The phase diagram of [N4111][TFSI]-propanol system was constructed based on visual cloud-point determinations [14] [15] [17] . The cloud-points of 1- and 2-based mixtures are represented by red and blue closed circles, respectively. On the phase diagrams, different molecular interactions of 1- and 2-propanol was predominant, since phase separation curves are calculated using the UNIQUAC interaction parameters [15] . The UNIQUAC model has the nearest neighbor correlation. The UCST of the [N4111][TFSI]-80 mol% 2-propanol mixture was approximately 15˚C. Therefore, below 15˚C, it is impossible to use nCO2 capture in the [N4111][TFSI]-2-propanol system for industrial applications.
Phase diagram including phase instability is connected with nCO2 capture ability in the propanol-rich region
![]()
Figure 4. (a) Temperature dependence of nCO2 molar fraction (η) in [N4111][TFSI]-80 mol% 2-propanol; (b) Phase diagram of the [N4111][TFSI]-propanol system.
(Figure 2). At the UCST, the phase separation behavior could significantly influence the nCO2 capture. Generally, liquid becomes unstable as a precursor phenomenon close to phase separation. Fluctuations in the propanol concentration in the vicinity of the clouding point were not ignored. Furthermore, UCST and the critical concentration (xc = 85 mol%) in the phase diagram have significant meaning on the UCST [15] . In thermodynamics, fluctuations are critical phenomena that are enhanced at UCST and xc. In this study, the intrinsic instability of the liquid phase in the RTILs-propanol mixtures became distinct at around xc. Thus, we deduce that the unstable liquid phase in the propanol-rich region was stabilized by nCO2 sorption.
3.3. TFSI− Conformation and nCO2 Capture
TFSI− conformation stabilities in the RTIL-propanol mixtures were estimated by Raman spectroscopy [15] [17] - [19] [22] . The observed Raman bands were assigned as C1/C2 conformers by DFT calculations [18] [22] . As an example, the Raman spectrum of pure [N4111][TFSI] is shown in Figure 5(a). The experimental C2/C1 ratio was calculated by decomposing the Raman peaks, and the asymmetric pseudo-Voigt function was used to separate peaks. The highest level of nCO2 capture for [N4111][TFSI] was obtained at C2/C1 = 0.539. In contrast to [N4111][TFSI], the C2/C1 values of [N1123][TFSI] and [P2225][TFSI], which exhibited poor nCO2 capture abilities, were 0.853 and 0.869, respectively. For instance, the Raman spectrum of [P2225][TFSI] is displayed in Figure 5(b). Despite pure systems, the C2/C1 ratio of TFSI− anion is regarded as a good indicator of nCO2 capture ability, and C2/C1 has been used to determine the mixing states of [Cnmim][TFSI]-propanol [15] and -bu- tanol [17] . In both the pure and mixed systems, the TFSI- anion conformer indicates energetically stable/unstable states in the liquid state.
DFT calculations are indispensable to interpret experimental results, although DFT calculations provide the molecular-level details on the gas phase. DFT calculations were performed using the Lee-Yang-Peer correlation (B3LYP) with the 6-31++G(d,p) basis set [23] [24] in the PC-GAMESS package [25] . In the DFT simulation box, we introduced the torsion angle (α) of TFSI− anion (Figure 6(a)); the geometrical definition of α was provided
![]()
Figure 5. Raman spectrum of (a) pure [N4111][TFSI], and (b) [P2225][TFSI] at room temperature. The decomposed peaks are assigned as C1 and C2 conformers of TFSI−.
![]()
Figure 6. (a) Definition of torsional angle α, (C-S-S-C) in TFSI−; (b) nCO2 molar fraction (η) as a function of calculated torsion angle.
by the C-S-S-C angle. DFT calculations of the [N4111][TFSI] system were used to examine a relation between a of TFSI− and molecular configurations of propanol isomer and CO2 additive (Table 1). The calculated torsion
angle is sensitive to the presence of propanol and its conformation. In pure [N4111][TFSI], α = 30.756˚; the addition of 2-propanol increased the torsion angle to 72.517˚. The effect of 2-propanol on torsion angle was larger than that of 1-propanol. These results are in agreement with previous DFT calculations. The experimentally obtained C2/C1 ratio of TFSI− anion is known to reflect the stabilities of the liquid, glass, and solid phases [14] [15] [17] [26] . Thus, increased α in the [N4111][TFSI]-2-propanol mixture has significant implications for liquid state stabilization. The torsional potential of TFSI− anion has two local minima at 80˚ and 280˚ [27] . Although the potential calculated between the two minima is relatively low, TFSI− has a higher torsional barrier at approximately 0˚ (C1). Hence, in case of the [N4111][TFSI]-2-propanol mixture, 2-propanol causes the TFSI- anion to twist, and stabilizes energetically. In the [N4111][TFSI]-1-propanol system, α cannot reached to 70˚. The difference in α between the 1- and 2-propanol-based RTILs is directly connected to the propanol isomer effect on nCO2 capture. To clarify the nCO2-driven stabilization in the RTIL-2-propanol systems, we replotted the observed CO2 capture against the calculated α angle (Figure 6(b)). The most nCO2 capture in the [N4111]- [TFSI]-2-propanol was observed at α = 70˚. In contrast, the minimum nCO2 capture in the [N1123]-[TFSI]-2- propanol system, which is fully stabilized without the addition of 2-propanol, was shifted to lower value of α. The α dependence of η in Figure 6(b) can be explained by assuming that CO2 compensates for geometrically mismatching of TFSI− conformer and additives in 2-propanol based mixtures.
3.4. Gas Selectivity of [N4111][TFSI]-2-Propanol
For actual applications, the RTIL-propanol mixtures must be gas selective. Figure 7 shows CO2, O2, and N2 sorption in the [N4111][TFSI]-80 mol% 2-propanol system at 25˚C. N2 cannot contribute to energetic stabilization in an unstable liquid system in the propanol-rich region. N2 mostly occupied in the air has the lowest sorption.
![]()
Table 1. Calculated torsion angle (α) of TFSI− anion. α is strongly dependent on the presence of propanol and CO2.
![]()
Figure 7. Gas selectivity in [N4111][TFSI]-80 mol% propanol at 25˚C.
The gas selectivity in the [N4111][TFSI]-80 mol% 2-propanol system has an advantage for industrial applications. The results clearly show that CO2 is preferred in the [N4111][TFSI]-2-propanol system. CO2 selectivity was realized at ambient pressure. CO2 plays an important role for the high efficient CO2 capture system, although the mechanism remains unclear.
4. Summary
At ambient pressure and room temperature, nCO2 capture in quaternary ammonium-based RTILs is promoted by the addition of 2-propanol. The propanol isomer effect associated with nCO2 capture is revealed by the lack of enhancement in RTIL with added 1-propanol. The conformation of TFSI− is regarded as a good indicator of nCO2 capture ability, since TFSI− torsion angle is strongly correlated with the amount of nCO2 sorption. The increase in nCO2 sorption in the propanol-rich region is consistent with liquid instability near the UCST, as shown in the phase diagram. The [N4111][TFSI]-2-propanol mixtures provide both high nCO2 capture and gas selectivity.
Acknowledgements
We appreciate Dr. T. Takekiyo, Dr. M. Aono and Prof. Y. Yoshimura of National Defense Academy for helpful discussions.
NOTES
![]()
*Corresponding author.