Sulfate-Reducing Bacteria Impact on Copper Corrosion Behavior in Natural Seawater Environment ()
Received 30 January 2016; accepted 4 April 2016; published 7 April 2016

1. Introduction
Copper is a metal of wide utilization due to its good electrical conductivity and resistance to corrosion, which has supported its applications as conductor in electrical power lines, in electronic industry and communications. Because of its high thermal conductivity, copper is used in heat exchangers, heat conductors and related applications [1] . In the atmosphere, copper forms a resistant coating of corrosion products named patina, which protects the metal from the deterioration. Practically, these applications of copper happened in atmospheric conditions [2] - [6] . These materials are also used in seawater immersed (seawater piping and heat exchangers) and in coastal zones bearing the effect of an intense marine aerosol. However, the seawater is an excellent corrosive environment due to its wealth in terms of mineral pollutants and also of different kinds of bacteria [7] . The marine bacteria strains responsible of microbial influenced corrosion are generally from the sulfate-reducing bacteria group. The anaerobic biocorrosion is always associated with the presence of the malodors of hydrogen sulfur produced by sulfate-reducing bacteria [8] [9] . These bacteria have enzymatic systems witch participate in different steps of corrosion process. Their hydrogenases depolarize the metallic surface to solubilize the metal [10] - [13] . Then the electrons produced are transferred to sulfate which is reduced in sulfur to provoke the dissolution of the metal [14] [15] . This dissolution is facilitated by the excretion of extracellular polymeric substances (EPS) which affect on the metal [16] . Compared to atmospheric patina, the layer of corrosion products formed under immersion conditions in seawater shows a poor protection to the metal [17] . The biofilm developed by D. desulfuricans at the metal surface accumulates with exposure time [18] , and the biofilm heterogeneities are responsible of local gradient differences and extension of the active sites where corrosion processes take place [19] [20] .
In this contribution we are reporting the results of a study on the corrosion of copper in seawater of coastal of Agadir from four different stations.
2. Materials and Methods
2.1. Metal Coupon and Medium Preparation
The phenomenon of copper corrosion in seawater was studied at four different marine stations: the first of them is the beach of Agadir, the second is industrial zone Anza, the third is the port, and the last is the Aghroud zone (Figure 1). These sites attempt to be representative of the seawaters of Agadir, and different in the type of industrial activity and wastes. Table 1 reported the values of some physic-chemical parameters of the seawater collected.
![]()
Figure 1. The marine zones of seawater collected: (a) Beach (Z1); (b) Port (Z2); (c) Anza (Z3) and (d) Aground (Z4).
![]()
Table 1. Physico-chemical properties of four zones of Agadir coastal.
The samples were prepared using copper 99.99% (weight percent), which was cut from a rectangular copper rod, with a total area of 5.68 cm2 for the electrochemical and gravimetric tests. Before the electrochemical measurements, the surface of copper was abraded using different grades of sand papers, which ended up with the 1200 grade. Then, the electrode was cleaned by washing with distilled water, acetone, distilled water, respectively, and immersed into the test solution quickly. The microbiological influenced corrosion was studied using a selective medium named Starkey medium in order to look for the reducing sulfate-bacteria culture. This medium is composed by: Na2SO4 (4 g), MgSO4∙7H2O (2 g), NH4Cl (2 g), KH2PO4 (0.5 g), yeast extract (1 g), Fe and Ca (traces), sodium Lactate (60%) (10 ml) and distilled water (1000 ml). The pH of this media was adjusted at 7.2 by the addition of 10 M KOH solution. After that the medium is sterilizing at 121˚C for 15 min.
2.2. Immersion Conditions
A conventional three electrode cell was used for all the electrochemical measurements. A saturated calomel electrode (SCE) was used as a reference electrode, platinum electrode acts as a counter electrode and the test material as the working electrode. Natural sea water collected from the coastal area of Agadir and synthetic seawater, at different concentration of sulfide ions, served as the electrolyte. Open circuit potential (OCP) and EIS measurements were carried out using Volta lab PGZ 301 Electrochemical Analyzer under computer control. The EIS experiments were realized in the frequency range from 100 kHz to 10 mHz at Eocp.
The charge-transfer resistance (Rt) values are calculated from the difference in impedance at lower and higher frequencies, as suggested by Tsuru et al. [21] . The double layer capacitance (Cdl) and the frequency at which the imaginary component of the impedance is maximal (−Zmax) are found as represented in Equation (1):
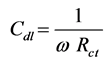 where
where 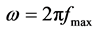 (1)
(1)
In order to test the reproducibility, the experiments were performed in triplicate. Cyclic voltametry was carried out for copper electrode in the natural seawater solution. The working electrode is scanned from negative to positive values in the potential range of −600 mV to 400 mV at a scan rate of 20 mV∙s−1. Additionally, other samples are prepared for the Atomic absorption measurement in order to follow up the evolution of the copper ions in the seawater solution at different durations. In this work, we are reporting to studying the corrosion of copper in the sea water in a long term. The maximal during is three months. After each 15 days, we proceed to the bacterial, electrochemical and gravimetric tests to follow up the corrosive behavior of copper and its dissolution in the corrosive medium. The corrosion rate is calculation by:
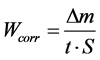 (2)
(2)
where Δm is the average weight loss before and after exposure, respectively; S is the surface area of sample and t is the exposure time.
2.3. Optical Microscopy Measurements
Immersion corrosion analysis of copper sample in the natural seawater solutions was performed using optical microscopy (OM). Immediately after the corrosion tests about three months, the samples were subjected to OM studies to examine the surface morphology. OM East Scope was used for the experiments. The working sample was analyzed at three different locations to ensure reproducibility.
3. Results and Discussion
3.1. Open Circuit Potential
The results of OCP variations are shown in Figure 2. In all marine zones, the OCP values associated to copper shifted to negative ones, but there are some differences which appear by increase in the immersed time. As seen in Figure 1, the OCP values associated to copper in Z1 seawater are more negatives, they begins at −230 mV and shifted at the end into −353 mV. However, the OCP values of copper in Z4 seawater are noble witch variated between −235 mV and −186 mV. But the copper takes a similar OCP values in Z2 and Z3 seawaters, they are comprise at −200 mV and −300 mV. Decrease in OCP is an indication of alloy nobleness deterioration and surface activity acceleration [22] .
![]()
Figure 2. Open circuit potential variations of copper in seawater of four zones of Agadir coastal.
3.2. EIS and Atomic Adsorption Results
EIS was used to investigate the electrochemical properties of the corroded surface after immersion of copper in naturel seawater of different marine zones for 15 days. This technique leads also to compare the corrosion rate of copper in different immersion period and studied marine zones. In fact, the corrosion rate is proportional to the inverse of the Rt (1/Rt). Figure 3 presents the Nyquist diagram of copper immersed in natural seawater and the electrochemical parameters issues form EIS measurements are given in Table 2.
We observe that the resistance to transfer of charge changed with the marine zones. It takes a value of 18.81 kΩ.cm² for the Z1, 11.25 kΩ∙cm2 for Z3, 9.27 kΩ∙cm2 for Z2 and7.808 kΩ∙cm2 for Z4. Consequently, after 15 days of immersion, the copper surface was modified: the resistance to transfer of charge of copper in the zones 2 and 4 is less than in 1 and 3 zones. This result can be explicated considering that the charge of bacterial didn’t have yet a enough time to develop in the medium and the surface was covered by the corrosion products or substances containing in the corrosive media which form a film on the metallic surface. Table 3 and Figure 4 present electrochemical parameters issues from the impedance response and the corrosion rate obtained by atomic absorption of copper samples exposed to seawater media of four marine zones and their evolution with time for three months as a maximal immersion period.
The graph presented in Figure 4 is associated to the copper corrosion behavior in Z1 seawater; it appears that the inverse resistance to transfer of charge, calculated from the impedance diagrams, increases at the first 30 days to decreases after the second month and starts to increase for the last month and it appears that the corrosion rate follows the same evolution. This results means that in this marine zone, firstly the copper was dissolved in marine environment but after one month the metallic surface is covered by the corrosion products which protect the metal. However, by the comparison of the evolution of electrochemical and gravimetric parameters in Z2 seawater (Table 3), the inverse of resistance to transfer of charge increases at the first month, and after this period it decreases progressively until the end of the exposure time [23] - [28] . This is the industrial port of Agadir city, so it receives a considerable quantity of polluted substances and hydrocarbons issues from different activities and results of deterioration of materials immersed in the seawater of port zone.
As will be discussed, this maximal decrease in the corrosion rate value is attributed to the formation of a thick layer of corrosion products at the surface with a large amount of fouling to form a composite material. For short immersion times the metal surface is covered by a thin layer of copper oxide (Cu2O) of dark brown color together with an increasing coating of green patina [29] . The third column of the table 3is attributed to copper immersed in Z3 seawater. It shows that the corrosion rate and inverse of the resistance to transfer of charge are very maximal at the second month of immersion. This graph describes the same evolution of growth cycle of sulfate-reducing bacteria (SRB) obtained in Anza zone for three month of immersion (Figure 5). In this zone, the corrosion process is controlled by the microbiology influenced corrosion MIC, provokes by the SRBs. Those bacteria are able to reduce the sulfate contained in seawater environment into sulfide and to product extracellular
![]()
Figure 3. Typical Nyquist plots of copper after 15 days of exposure to different natural seawater.
![]()
Figure 4. The evolution of the inverse of charge transfer resistance and corrosion rate of copper in Z1 seawater under immersed conditions.
![]()
Table 2. Electrochemical parameters of copper corrosion immersed for 15 days in seawater of the four marine zones of Agadir coastal.
![]()
Table 3. Electrochemical parameters and corrosion rate values of copper samples exposed to different seawater media.
polymeric substances (EPS) which leads to the dissolution of the copper [29] . This mechanism needs a time to the bacterial proliferation and metabolic stimulation. The last column in Table 3 shows that the corrosion rate and the inverse of resistance to transfer of charge the copper immersed in Z4 seawater present two maximal peaks: at the first and second months of immersion. The corrosion rate evolution in this zone is different than other zones because the values of the corrosion rate for Z4 seawater are less. It is the reason of the appearance of two maximal peaks of the rate. In addition, the copper surface immersed present a few corrosion products, and it isn’t enough covered and protected by the patina layer. All this results are compatible with the nature of each zone and its pollution level and showed that the biofilm produced by the sulfate-reducing bacteria accumulates with the exposure time especially after two month of exposure [30] .
Figure 5 translates the evolution of corrosion rate of copper estimated by the both methods electrochemical and gravimetric in different marine environment after 15days of under immersion conditions. We notice that we have a good correlation between the results obtained by the methods used. We observe the corrosion rate and the inverse of the charge transfer are more important in Z4 and Z2.
Cyclic voltammograms (CV), copper electrode in natural seawater at 20 mV∙s−1 are presented in Figure 6 and Table 4. It can be observed that two oxidation peaks in forward scan and one large reduction peak in the reverse scan. The peak a1 is related to the formation of CuCl salt layer [31] . However, the second oxidation peaks (a2) corresponds to oxidation of Cu+ into Cu2+. The large reduction peak corresponds to the reduction of soluble 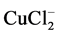 complex and the CuCl layer formed on the copper surface. By the analysis of potential and current values of CV curves, we observed that the first oxidation of Cu to Cu+ is important in the Z3 compared to other zones. It is in agreed with the microbiological tests revealed that this zone was polluted by the sulfate-reducing bacteria which reduces the sulfate to sulfide which participate at the metal dissolution.
complex and the CuCl layer formed on the copper surface. By the analysis of potential and current values of CV curves, we observed that the first oxidation of Cu to Cu+ is important in the Z3 compared to other zones. It is in agreed with the microbiological tests revealed that this zone was polluted by the sulfate-reducing bacteria which reduces the sulfate to sulfide which participate at the metal dissolution.
![]()
Figure 5. The evolution of the inverse of charge transfer resistance and corrosion rate of copper immersed in different marine zones for 15 days.
![]()
Table 4. Electrochemical parameters obtained from CV of copper in seawater of the four marine zones of Agadir coastal.
![]()
Figure 6. Cyclic voltammograms of Cu in natural seawater of different marine zones.
3.3. Microbiological Analysis
The microbiological analysis concerns the count of sulfate-reducing bacteria in the seawater of Agadir coastal. The results of this analysis are represented in Figure 7 and Table 5. Seawater samples are collected from four different marine areas.
The microbiological tests realized shows that the Beach, the Port and Aground zones present the absence of all kind of sulfate-reducing bacteria. However, the Anza zone shows the present of a considerable charge of sulfate-reducing bacteria as known as the seawater is collected near than an area when is rejected the waste water of the city. This charge varies with the immersion time, it increases when the immersion begins until 75 days and it decreases after. The growth takes a maximal value at the 60th day. This evolution is the cycle of the life of this bacterial group. It is logically that this group acquires enough time to adapt with the environment on searching different sources of carbon, sulfate, sodium and other important elements for its growing and development. After this step, it moves to attain maximal proliferation at optimal conditions (pH and elements) by consuming the elements containing in the medium which will be exhausted to decrease the bacterial charge [31] [32] .
3.3.1. Correlation between Microbial Growth of Sulfate-Reducing Bacteria and Copper Corrosion Rate
The microorganisms are easily adhered on the surface of different materials [33] . If the metal is immersed in seawater, the organics fragments attached on material surface to form thin films. This film changes the characteristics of the metals surface witch become a favorite medium to attract the bacteria and help to their growth to form colonies. This film is known by biofilm and contains the diatoms, funguses, protozoa, microalgaes and their metabolite products [33] . In our study, we found that the sulfate-reducing bacteria increase the copper corrosion rate under immersion conditions after two months in the Z3 (Figure 8): a sufficient duration to develop and produce the different metabolite products in the environment. The mainly products are the sulfide witch form CuS or Cu2S. This figure shows a good correlation between the corrosion rate and the evolution of SRB growth [33] . By this result, we propose that in two months of immersion, the quantity of sulfide produced by SRB react with ions of copper dissolved to form CuS or Cu2S at the copper surface and increase the metallic dissolution. But at the last month of immersion, we have the diminution of the bacterial activity because of the exhaustion of the necessary elements which need the bacteria to its metabolism. This decreasing of bacterial activity was accompanied by the decreasing of the corrosion rate of copper. This result translates the effect of the exposure of copper surface at the different pollutants and aggressive agents in natural seawater such as the chlorides and sulfides ions, the microbial elements and theirs metabolites and this also linked to a decrease in pH in
![]()
Figure 7. The growth cycle of SRB in natural seawater of four marine zones of Agadir coastal.
![]()
Figure 8. The evolution of corrosion rate of the copper in seawater of different marine zones of compared with the growth cycle of SRB under immersed conditions.
![]()
Table 5. The count of sulfate-reducing bacteria in natural seawater of Agadir coastal.
the medium in the presence of hydrogen sulfide (sulfate-reducing bacteria, nitrate-reducing bacteria, iron-oxi- dizing bacteria…) [30] . Those entire elements participate on the destruction and corrosion properties of the naturel marine environment. In other hand, the synthetic seawater contains only the ions and it is sterilized before each utilization. So, in this case, the corrosion mechanism is only affected by the cotenants of the solution.
In other hand, in the absence of SRB (Z1, Z2 et Z4) we steel have the maximal pics associated to corrosion rate because the natural seawater is an enriched environment: it contains also the chlorides ions witch accelerate the corrosion mechanism by initiating of the pitting corrosion on of copper in marine environment.
3.3.2. Microbial Effect on the Formation of Green Rust at Copper Surface
In order to visualize the copper surface immersed in natural seawater of different marine areas, we used the optic microscopy. The images presented in Figure 9, indicated that the green rust density formed at the copper surface changed according to kind of marine zone. We observed that surface immersed in Z3 seawater present a high quantity of green rust; this area corresponds to the highest density of SBR. In fact, we can suggest the sulfate-reducing bacteria motivated the production of many corrosion products. This study proposed that the sulfate-reducing bacteria in anaerobic biofilms participate in the corrosion and rust mineralization of copper in natural seawater. In laboratory conditions, we simulate seawater; electrochemical measurements and atomic absorption indicated that the SRB accelerated or inhibited corrosion mechanism depending on the availability of necessary elements for SBR growth and on the immersed time. Antecedent studies and our present study show that the green rust is the main component of the inner rust layer. The middle and outer rust layers are mainly made of copper oxides. In many studies with a single strain, it has been observed that the copper sulfides are the main corrosion products, suggesting that the copper sulfide is converted to green rust [33] .
By the analysis of the different results occurred in this study, we can simulate the surface of copper as shown in Figure 10. The SBR bacteria act by different mechanism like the sulfate reduction, metabolic products (acide, polymers…). Then the copper surface becomes a favorite medium to another microorganism. In addition, the organic and inorganic elements can attached at the surface to form a SBR-Biofilm. This biofilm layer can act as corrosive or inhibitive layer depending on the environnement conditions.
![]()
Figure 9. The optic microscopic images of copper surface under immersed conditions in different marine zones.
![]()
Figure 10. Schematic representation of corrosion mechanisms by biofilm-forming of the sulfate-reducing bacteria.
4. Conclusions
To study the effects of sulfate-reducing bacteria on the resistance to corrosion of copper in marine environment, microbiological analyses, the open circuit potential, the electrochemical impedance measurements and the atomic absorption tests are investigated. These various tests led to the following results:
OCP results indicate that the potential of copper immersed in Z4 seawater is nobler than other marine zones.
EIS and atomic adsorption mentioned that the corrosion rate changed with the immersed time depending on the marine zone. But after two months, it decreases because of accumulation of corrosive products and microbial biofilm on the copper surface.
The comparison between the electrochemical behavior of copper in natural and synthetic seawater reveals that copper resistance is greatly high in synthetic seawater than the first one.
Following the evolution of SBR growth allow to identify the steps of SBR life cycle. The SBR effect on the copper corrosion is accelerated but they can produce a protective layer on copper surface.
NOTES
![]()
*Corresponding authors.