Received 5 January 2016; accepted 12 February 2016; published 17 February 2016

1. Introduction
Many authors were interested in the study and preparation of the new double perovskite materials. The general chemical formula of double perovskite oxides was expressed as A2BB’O6, where A is occupied by an element of group one or group two such as Ca, Sr or Ba and B, B’ site is occupied by a translational element, while the O atom is located in between, forming the alternate BO6 octahedral and B-O-B bonds. The wide variety of double perovskite materials is due to the alternation of the magnetic and non-magnetic B and B’ elements [1] [2] . In particular, the compositions A2BMoO6, where B = Fe, Mn or Cr are magnetic ions, are currently studied for their potentiality as magnetoresistive and thermoelectric systems [3] .
Synthesize of Sr2CaWxMo1-xO6 double perovskite series by solid state reaction was carried out by Zhou et al. [4] . The self-activated host material emits strong blue lights at round 435 nm and 468 nm, while the excitation emits at or near UV at about 290 nm due to W-O charge transfer transition. Tian et al. [5] studied the crystal structure and magnetic properties of Sr2MWO6 (M = Co or Ni), using sol-gel method, showing tetragonal (I4/m) structure and homogeneous grain size. The magnetic parameters of the material refer to paramagnetic behavior. The magnetic momentum originates mainly from the interaction between the Ni2+ ions and Co2+ ions in Sr2NiWO6, Sr2CoWO6 respectively. Manoun et al. [6] investigated the effect of temperature in the structure of Sr2ZnWO6 and Sr2CoWO6 double perovskite oxides when characterized by Raman spectroscopy. The first phase transition from monoclinic (P21/n) crystal structure to tetragonal (I4/m) crystal structure occurred at 80˚C, while the second transition to cubic (Fm-3m) occurred at 480˚C. The effect of the preparation methods on the catalytic activity of the LaSrFeMo0.9Co0.1O6 methane combustion double perovskite oxide was studied by Zheng et al. [7] using two methods; co-precipitation and sol-gel. The sol-gel method had greater impacts on the catalytic activity than co-precipita- tion method. The Ca2NiWO6 double perovskite oxide structure and magnetic behavior at 1150˚C with P21/n space group monoclinic crystal structure was investigated by Lopez et al. [8] . Ba2MgWO6 and Ba2ZnWO6 double perovskite oxides were synthesized using the solid state method by Bugaris et al. [9] . They characterized the samples by single-crystal X-ray diffraction, Neutron diffraction, UV-visible spectrometry and LS 55 Fluorescence Spectrometer. The cubic (Fm-3m) crystal structure was obtained.
In this work, the Ba2Znx−1NixWO6 double perovskite oxides were synthesized using solid state reaction method. The aim was to investigate the effect of replacement of Zn2+cation with Ni2+ cation. The X-ray diffraction (XRD) and Fourier Transform Infrared Spectroscopy (FTIR) were used in order to explore the effect of replacement on the structural properties of Ba2Znx−1NixWO6 double perovskite oxides.
2. Experimental
The new samples of double perovskite oxides were synthesized using solid state interaction method and many different treatments were applied in order to get single phase for the samples. The samples were prepared by mixing stoichiometric amounts of ZnO, NiO, WO3 and BaCO3 and these chemicals were purchased from Alfa Acer of purity 99.9% in order to prepare the Ba2Zn1-xNixWO6 double perovskite oxides. The mixtures of compounds grinded in agate mortar with the addition of acetone and then kept in crucibles and heated in air at 800˚C for 12 hours. The samples pellet in around shape and heated in at 1000˚C and 1200˚C, respectively. Every step of heating treatment was repeated twice with the rate of 10˚C per minute during the heating process and coaling as well. The compound permeated every time grinding with the addition of acetone. The ratio of the amounts calculated was by the following equation:
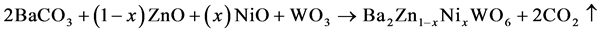 (1)
(1)
The X-ray diffractometer (Model Bruker D-8) using Cu-Kα radiation ( 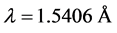 ) and a nickel filter operating at 40 KV and 40 mA at scanning the samples was used at room temperature. The data were collected for the 2θ range 20˚ - 80˚ at step size of 0.02 and count time of 5 s. The collected data were then fed to full Prof suite [10] for determination of the lattice parameters, space group, atom’s positions and crystalline size (D) using Scherer equation [11] .
) and a nickel filter operating at 40 KV and 40 mA at scanning the samples was used at room temperature. The data were collected for the 2θ range 20˚ - 80˚ at step size of 0.02 and count time of 5 s. The collected data were then fed to full Prof suite [10] for determination of the lattice parameters, space group, atom’s positions and crystalline size (D) using Scherer equation [11] .
 (2)
(2)
where D is crystallite size, λ is the wavelength of X-ray and β1/2 is the half full width of the peaks.
The tolerance factor [12] [13] for the samples calculated by
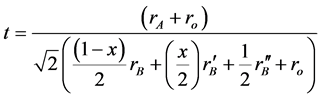 (3)
(3)
A Fourier transform infrared spectroscopy (FTIR) spectrum was recorded in transmittance mode at room temperature using KBr pellet method, the samples were diluted in KBr of ratio 1:100 for FTIR measurement between 400 and 2000 cm−1 the FTIR of samples were carried out with a (Satellite FTIR 5000 of the wavelength range of 400 to 4000 cm−1) [14] where the characteristic bands and peaks of perovskite structure can be assigned.
3. Results and Discussion
3.1. The XRD Results
The study and determination of the structural properties of perovskite oxide compounds are very important; such as lattice parameter, space group, type of crystal, atomic position, and molecular binding because they predict and expect the electrical, optical and magnetic properties of materials [15] . The X-ray powder diffraction obtained for Ba2Zn1-xNixWO6 where (x = 0, 0.25, 0.50, 0.75, 1) double perovskite series prepared by the solid state reaction is shown in Figure 1. The difference in lattice parameters for each sample is shown by the different peaks position in Figure 2. The shift in reflection positions is referred to the differences in lattice parameters of unit cells of the samples. The peaks appeared at low intensity in the XRD pattern shown in Figure 1 are attributed to (Ba2WO5/Ba2WO4) impurities following Manoun et al. [16] .
3.2. XRD Refined Result
The XRD data of each sample of series are refined by Retiveld method. All samples obtained are showed cubic structure with (Fm-3m) space group. Table 1 shows the atom positions, the space groups and the lattice parameters of the samples. Figures 3(a)-(e) show the refined XRD patterns of the Ba2Zn1-xNixWO6 double perovskite series where x = 0, 0.25, 0.50, 0.75, 1. In the case of Figure 3(c), the XRD refinement of Ba2Zn0.50Ni0.50WO6 is shown, which represents (Fm-3m) cubic structure with a = b = c8.097554 Å, α = β = γ 90 lattice parameters with x = 0.50. It is clear that the shift is dependent on the ratio of replacement. Bugaris et al. [9] found similar results
![]()
Figure 1. X-ray powder diffraction of Ba2Zn1-xNixWO6 double perovskite oxides series.
![]()
Figure 2. XRD pattern for selected range of 2θ.
![]()
Table 1. The atom positions, the space groups, the lattice parameter and crystallite sizeof the Ba2Zn1-xNixWO6 double perovskite series where (x = 0, 0.25, 0.50, 0.75, 1).
for the Ba2MWO6 double perovskite samples where (M = Zn, Mg) using the Single-crystal X-ray diffraction and Neutron diffraction. Furthermore, Y. Tamraoui et al. [17] found the similar structure (Fm-3m) cubic structure for Ba2−xSrxMgTeO6 series where x lies between 0 and 1.5.
From X-ray result, the cubic structure is confirmed for all samples so the Ba-cation is surrounding by 12 oxygen anions in a regular dodecahedral environment and the Zn-cation, Ni-cation, W-cation is octahedral coordinated by six oxygen ions, giving rise of 180˚ B-O-B bond angles [18] [19] . The crystallite size for Ba2Zn1-xNixWO6 double perovskite series is calculated from full-width at half- maximum (FWHM) using Scherer formula for the major peaks at (220) that observed to vary between 71.91 to 148.71 nm for the samples. The tolerance factor is calculated and is found to be between (1.008 - 1.020) as shown in Table 1. This is confirmed the (Fm-3m) cubic structure according to the criteria adopted by Correa et al. and Popov et al. [12] [13] . Figure 4(a) and Figure 4(b) show the relation between the tolerance factor and volume of unit cell respectively with ratio of replacement. The tolerance factor of the samples is found to take almost the same value. This may be attributed to the same values of ionic raduii of Ni2+ and Zn2+ cations. D. Serrate et al. [20] verified the rule of double perovskite tolerance factor for the (Fm-3m) cubic structure to be between 1.05 - 1.00. The volume of unit cells is found to decrease with the increasing ratio of replacement ofZn2+ cations by Ni2+ cations. This is may be due to the size of Zn2+ cations being larger than the size of Ni2+ cations.
3.3. The IR Spectroscopy Results
The FTIR spectra of the perovskite structure have three characteristic absorption bands between 850 - 400 cm−1, respective to composition and these are usually used to identify the perovskite phase formation [21] . It is based on infrared spectrum can be used for molecules such as fingerprint for humans [22] . The strong high-energy band centered at about 620 cm−1 can be assigned to the anti-symmetric stretching mode of the (Zn-O6), (Ni-O6) and (W-O6) octahedral is due to the higher charge of this cation. Another interesting point appear by this spectra is the presence of the high intensity band at about 825 cm−1 which can be assigned to the symmetric stretching vibration of these octahedral as explain. The positions of these bands suggests relatively long (Zn-O6), (Ni-O6) and (W-O6) bands [23] [24] . The bands appeared around 420 cm−1are may be due to deformational modes of theZnO6, NiO6 and WO6 octahedrals. From Figure 5 that shows the transmittance of Ba2Zn1-xNixWO6 double perovskite series versus wave number; all the samples confirmed the molecular bands on the form perovskite oxide [24] . The peak between 1450 - 1400 cm−1 is likely corresponds to overtones of the fundamental vibrations of carbonate [25] [26] .
![]()
Figure 4. (a).The tolerance factor versus the ratio of replacement; (b) The volume of unit cell versus the ratio of replacement.
![]()
Figure 5. The IR spectra of theBa2Zn1-xNixWO6 double perovskite oxides.
4. Conclusion
The Ba2Zn1-xNixWO6 double perovskite series, where x = 0, 0.25, 0.50, 0.75, 1, were synthesized using solid state method. The X-ray diffraction (XRD) and the Fourier Transform Infrared Spectroscopy (FTIR) were used as analytical tools. The XRD patterns were measured at room temperature for the series and showed the same cubic structure before and after the replacement of Zn2+ cation by Ni2+. As a result of a convergence of the ionic radius of the Zn2+ cation and Ni2+ cation, the unit cell volume was found to decrease from 535.059 nm3 to 527.648 nm3, while the values of tolerance factor were found to slightly increase from 1.008 to 1.02. FTIR absorption bands were found to be characteristic of perovskite structure.
Acknowledgements
This work is supported by Materials Laboratory of Alneelain University, Khartoum, Sudan.