Assessment of Heavy Metals and Radionuclide Concentrations in Mafikeng Waste Water Treatment Plant ()
Received 18 June 2015; accepted 22 January 2016; published 25 January 2016

1. Introduction
Water is a natural resource that forms an integral part of any ecosystem. Access to clean water is a key resource for reducing poverty and disease, and improving the life of any human population [1] . South Africa is a country plagued with unpredictable rainfall (which averages 500 mm/a), high evaporation rates and low conversion of rainfall to runoff. These shortcomings make South Africa a water-stressed nation, where demand is almost closing in on supply. The North West Province, where Mafikeng is located, is an arid province that experiences all of the aforementioned water resource constraints. The Mafikeng community water supply is sourced mainly from surface water (Setumo dam) and groundwater (Molopo and Grootfontein well-fields), both of which are rapidly depleting due to insufficient rainfall, agricultural/industrial and mining activities [2] . The water resource constraints are further compounded by quality deterioration due to pollution accruing from domestic sewage, industrial effluents, acid mine drainage and agricultural runoff chemicals (fertilizers, pesticides and herbicides). Naturally, groundwater contains varying concentrations of radioactive metals such as uranium, thorium and their daughter products [3] . Also, each aforementioned pollution point-source has the potential to raise the levels of concentration of heavy metals and radionuclide contents of surface water. Both water resource bodies, if not properly treated to remove radionuclides and heavy metals, may contribute significantly to internal radiation dose through drinking water. In addition, the current Mafikeng water consumption exceeds the calculated required needs, necessitating a cycle of water re-use [4] (Figure 1). This makes water treatment an issue of paramount importance in the locality, if public health must be protected. A few functioning wastewater (WWTW) and water treatment (WTW) infrastructures are located within the municipality (Figure 2). However, the quality
![]()
Figure 1. Mafikeng local municipality water recycle system (adapted from [4] ).
![]()
Figure 2. Map of Mafikeng local municipality showing locations (indicated by drop) for water treatment infrastructures. MWWTW = Mafikeng waste water treatment works.
2. Materials and Methods
2.1. Sampling Area
The North West Province is located on latitude 25.8˚S and longitude 25.5˚E and offers an almost year-round sunshine, with average rainfall of 300 - 500 mm annually. This study was carried out within the Mafikeng Local Municipality (MLM), the biggest municipality in the Province which covers an area of about 3703 km2 and consists of 102 villages and suburbs. Economic activities within the municipality consist of agriculture, agro-industries and tertiary sectors.
2.2. Sample Evaluation of Heavy Metals
The concentration of heavy metals in the water samples was determined using the Inductive Coupled Plasma Mass Spectrometer (Perkin Elmer NexION 300Q) located at the Animal Health Centre of the North West University, Mafikeng Campus. Prior to analysis, samples were digested as described by [5] with modifications. To 5 ml aliquot of samples, 5 ml of 55% HNO3, 5 ml of 32% HCl and 1 ml of 100 vol. H2O2 were added, followed by heating in a microwave for 45 min. The mixture was allowed to cool for 20 min and later transferred to pre-rinsed 100 ml volumetric flask where it was topped with distilled water to make 100 ml. The mixture was left at room temperature overnight and thereafter transferred to 15 ml centrifuge tubes without disturbing the sediments.
2.3. Determination of Radionuclides Concentration in Water
Determination of gross alpha/beta activities in water samples was carried out by Liquid Scintillation Counting (LSC) using the Perkin Elmer Quantulus 1220 Ultra-Low Level LSC coupled to alpha-beta discrimination (Todorović et al., 2012). A 10 ml aliquot of each filtered and acid-preserved water sample was added to 8 ml of Ultima Gold ULLT Cocktail (Perkin Elmer, Boston, USA) in a 20 ml vial. The water samples were then run on the Quantulus 1220 Ultra Low-Level LSC set at 4 h counting time, 3 cycles and 1 repetition per sample vial. The radionuclide concentrations in samples were determined using HPGe detector model GC2020 E7500 CSL (Canberra GMbH) {resolution (FWHM) at 122 keV (57Co) is 0.94 keV and at 1332 keV (60Co) is 1.77 keV and relative efficiency for energy 1.33 MeV relative to (NaI)TI is 20%. The detector was coupled to a computer through an MCA (DSA 1000, Canberra). The detector was calibrated for energy and efficiency using the Canberra standard calibration file. Each sample was counted for 24 h. The Genie 2000 software was used for both Data acquisition and analysis (nuclide identification).
2.4. Estimation of the Annual Intake Dose
The annual intake dose (AID) of some important radionuclides through drinking water over one year period for all age groups was calculated for community water using the following equation [6]
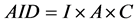 (1)
(1)
where I is annual water consumption (L/a), A is the activity concentration (Bq/L) and C is the dose conversion factor for each radionuclide. The dose conversion factors and annual water consumption (L/a) for different age groups was extracted from DWAF (2002) guidelines [7] . The total annual dose was calculated as the arithmetic summation of annual intake dose of two radionuclides (235U and 226Ra).
3. Results and Discussion
3.1. Concentration of Heavy Metals in Water Samples
The heavy metal concentration of analyzed water samples is shown in Table 1. The values of all the metals detected in samples obtained from the Mafikeng WWTW were within limits stipulated by the Department of Water Affairs (DWA) and World health Organization (WHO) although there were staggering variations in values at different processing stages. This could be due to effects of treatment. However, most of the metals were not detected in the water out samples (i.e. effluents released to the Setumo dam) except vanadium, iron and arsenic which were also far below stipulated limits. This observation is a suggestion that the water treatment carried out at the WWTW may be very efficient in removing the heavy metal contents of the wastewater delivered to it.
3.2. Gross α/β Concentrations of Water Samples
3.3. Radionuclide Concentrations of Water Samples as Determined by Gamma Spectroscopy
The trend observed in the concentrations of both Uranium-235 and Radium-226 radionuclides from waste water entering the treatment plant (235U = 9.75 Bq/L and 226Ra = 5.44 Bq/L) to the effluent (235U = 16 Bq/L and 226Ra = 5.56 Bq/L) in Table 3 may be an indication of poor efficiency of the employed water treatment method in removing the radionuclides. This could cause the respective radionuclides which naturally have long half-lives (235U = 700 million and 226Ra = 1600 years) to accumulate in the system over time leading to further contamination of water during processing. Furthermore, it was previously noted that effluents delivered into the Setumo dam (source of water for domestic use) are of poor quality, and which eventually reduces the efficiency of the final water treatment at Mmbatho water treatment plant [11] . This could be a contributing factor to the exceedingly high concentrations of the radionuclides in the community water (235U = 73.8 Bq/L and 226Ra = 47 Bq/L). This is in addition to probable contamination of the community water delivery system as a result of previous radionuclide accumulations, which in turn may contaminate the delivered water. It has been reported that most water treatment methods generate waste products containing concentrated radionuclides which if not properly handled or treated, become a source of radiation in itself [12] . However, the studies of [3] in South India showed that water treatment by reverse osmosis reduced the concentrations of natural radionuclides in drinking water. The high activity concentration of uranium and radium nuclides observed in this study agrees with reports of [13] , who also noted high dose contributions of radium (48.0% ± 27.9%) and uranium (20.3% ± 14.1%) in drinking water in Italy. Evaluation of activity concentration of 226Ra in drinking waters from oil fields and host communities in Nigeria revealed average concentration that was well above the WHO permissible levels [6] . Uranium and radium are natural radionuclides of practical importance in terms of drinking water due to their effects on health. Radium tends to accumulate in the bony skeleton leading to increased risk of bone cancer particularly when the activity concentration is above 0.42 Bq/L. Uranium has affinity for kidneys and liver making its chemical toxicity to be of greater concern than the radiological cancer risk. Short term risk of renal damage may occur with activity concentration of uranium greater than 18 Bq/L of drinking water [8] . The estimated annual intake dose and the total annual radiation dose (μSv∙yr−1) resulting from consumption of 235U and 226Ra in drinking water are presented in Table 4.
Despite the high activity concentrations of the two natural radionuclides in the community water, the estimated total doses for the analyzed water samples were all well below the reference level of the committed
![]()
Table 1.Heavy metal concentration of water samples as measured by infuctive coupled plasma mass spectrometer(µg/L).
![]()
Table 2.Concentrations of gross α/β activity in water samples as measured by liquid scintillation counter(Bq/L).
![]()
Table 3.Radionuclide concentration of water sample by HPGE-gamma spectroscopy(Bq/L).
![]()
Table 4. Estimated intake dose (μSv∙yr−1) and total annual dose of 235U and 226Ra from drinking water in different age groups.
effective dose of 100 μSv∙yr−1 recommended by WHO [14] . This is an indication that the community water analyzed may not pose a radiological risk to the consumers.
4. Conclusion
Results of the evaluation of heavy metal and radionuclide concentrations of water from the Mafikeng waste water treatment plant and the community water suggest that the water treatment method employed may be efficient in removing heavy metals from sewage delivered to the plant but not the radionuclides particularly 235U and 226Ra. The increasing trend in the concentrations of these radionuclides from the delivered sewage to the effluent and finally to the end users may suggest contamination along water processing line and the final water delivery system. Despite this observation, the community water analyzed may not pose any radiotoxic risk to the community because the estimated total annual dose from analyzed water samples was far below reference level recommended by World Health Organization. However, improvements in the methods and facilities currently used for water treatment at the Mafikeng WWTW and proper care of the community water delivery system may go a long way in improving the quality of water delivered to the Mafikeng people.
Acknowledgements
The authors would like to thank the NRF and FAST FRC for providing the funds for this research, and also Ms. Mpho Tfheole for her assistance during the laboratory analysis of samples.
NOTES
![]()
*Corresponding author.