Received 4 December 2015; accepted 21 December 2015; published 24 December 2015

1. Preface
Paraffin wax deposition issues have a significant impact on oilfield production. The deposition of paraffin wax in the tubing and pipe can reduce the production efficiency. In the meantime, removing the deposition of paraffin wax will increase a lot of expenses. The process of paraffin wax deposition is a very complicated problem. On one hand, because the composition of the oil and gas system is very complex, there needs further research on the effect of various components on paraffin deposition. On the other hand, many theoretical issues are involved in the process of paraffin deposition as solubility, crystal, fluid dynamics, mass transfer dynamics, heat transfer, etc. The mechanism of paraffin deposition is not yet fully understood; there are many kinds of explanation theory as solubility theory, crystallization theory, diffusion theory, and phase equilibrium theory [1] . At present, phase equilibrium theory was widely accepted. According to this theory, reason of paraffin wax deposition is the thermodynamic condition of oil and gas system, such as the change of oil and gas system composition, component, temperature, and pressure [2] .
Model established in this paper can be used to determine cloud point of paraffin wax and the deposited amount of paraffin with the lowering of the temperature, which has important reference value for oilfield to solve paraffin deposition problems.
2. The Thermodynamic Model
When the phase equilibrium of gas, liquid, solid phase is reached [3] .
 (1)
(1)
where
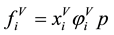 . (2)
. (2)
 . (3)
. (3)
 . (4)
. (4)
If considering the heat tolerance of the liquid and solid, fugacity of solid standard state and liquid standard state has the following relationship.
 . (5)
. (5)
where
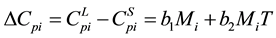 (6)
(6)
where b1, b2 get from the experimental fitting. In the absence of experimental data b1, b2 can get
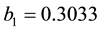 . (7)
. (7)
 . (8)
. (8)
Take (6) into (5), (5) becomes
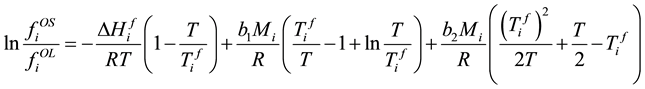 . (9)
. (9)
The non ideal of the solid mixture is corrected by using the regular solution theory, then derived the calculation formula for the activity coefficient of solid phase.
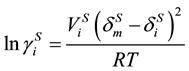 (10)
(10)
where
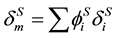 . (11)
. (11)
 . (12)
. (12)
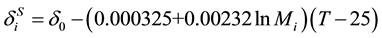 . (13)
. (13)
 . (14)
. (14)
 . (15)
. (15)
Use the Flory theory of polymer solution to correct the liquid activity coefficients, the calculation formula as follows [4] .
![]() (16)
(16)
where
![]() . (17)
. (17)
Molecular interaction is divided into three categories: (1) PN―PN (2) PN―A (3) A―A. Among which P stands for alkane, N stands for cycloalkane, A stands for aromatic. Their molecular interaction coefficients are as follows:
![]() . (18)
. (18)
![]() . (19)
. (19)
![]() . (20)
. (20)
Paraffin wax is mainly composed by the n-alkane, but contains a small amount of isoparaffin, cycloalkane and aromatic. For these non-normal paraffin, their melting point and other properties are very different from the n-alkane, the melting point of normal paraffin wax decreases obviously when a branch is added. For example: the melting point of C43H88 is 188˚F, it is generally believed that the melting point of C52H104 is relatively high. But because there is a branched chain in C52H104, its melting point will be lower than C43H88 nearly 100˚F, it’s 91˚F. Therefore, it is necessary to consider the effect of non-normal paraffin [5] .
For normal structure paraffin wax:
![]() . (21)
. (21)
![]() . (22)
. (22)
For non-normal structure paraffin wax:
![]() . (23)
. (23)
For isoparaffin, cycloalkane:
![]() . (24)
. (24)
For aromatic:
![]() . (25)
. (25)
3. The Example Calculation and Results
In order to validate the model, calculated by using the data of [6] (see Table 1) (SRK state equation is used in calculation). Calculation results are shown in Figure 1 and Figure 2.
From Figure 1 we can see that the cloud point temperature is different under different pressures. This difference is because of the combined effect of pressure and composition. Temperature decreases with increasing pressure. Under 1.5 MPa, the measured cloud point temperature is 318.7 K, calculating cloud point temperature is 318 k, measured and calculating results are quite close. It can be seen from the Figure 2 that the theoretical results are in good agreement with the actual results.
![]()
Table 1. The components of a kind of crude oil in the Beihai Oilfield.
![]()
Figure 1. The diagram of cloud point temperature.
![]()
Figure 2. The diagram of wax deposition content.
4. Conclusions
1) The model proposed in this paper is more accurate, and the prediction results are in good agreement with the practical data.
2) The model considers the non ideality of the solid phase mixture, and the activity coefficients of solid phase are corrected by using the regular solution theory.
3) The model considers the influence of the non-normal paraffin; it makes the results more accurate.
Funding
This work was supported by the National Natural Science Foundation of China (No: 51404072).
Explanation of Nomenclature
![]() fugacity;
fugacity;
![]() fugacity of solid standard state;
fugacity of solid standard state;
![]() fugacity of liquid standard state;
fugacity of liquid standard state;
![]() mole fraction;
mole fraction;
![]() activity coefficient;
activity coefficient;
![]() fugacity coefficient;
fugacity coefficient;
![]() molar enthalpy of fusion;
molar enthalpy of fusion;
![]() melting point temperature;
melting point temperature;
![]() specific heat under constant pressure;
specific heat under constant pressure;
![]() coefficient;
coefficient;
![]() molecular weight;
molecular weight;
![]() molar volume of component;
molar volume of component;
![]() solubility parameter;
solubility parameter;
![]() carbon number of component i;
carbon number of component i;
![]() mole number of component i;
mole number of component i;
![]() coefficient of molecular interaction;
coefficient of molecular interaction;
![]() pressure;
pressure;
![]() gas constant;
gas constant;
![]() temperature.
temperature.
Superscript:
![]() gas phase;
gas phase;
![]() solid phase;
solid phase;
![]() liquid phase;
liquid phase;
![]() standard state;
standard state;
![]() liquate.
liquate.
Subscript:
![]() pure component;
pure component;
![]() constant pressure.
constant pressure.