Received 9 November 2015; accepted 19 December 2015; published 22 December 2015

1. Introduction
The contamination of waterways, channels, and lakes with concoction substances of anthropogenic starting point may have unfriendly outcomes; the waters get to be unacceptable for drinking and other family purposes, watering system, and fish cultivation; furthermore the animal communities living in them may endure genuinely [1] [2] . Pesticides have turned into an inexorably serious wellspring of substance contamination of the earth because of their broad utilization in agriculture which can influence the freshwater fauna, especially fish [3] . Pyrethroids are synthetic compounds that mimic the pyrethrins that are detached from chrysanthemum blooms [4] . They have emerged as good substitutes for organochlorine, organophosphate and carbamate insecticides due to their high bio-efficacy, biodegradable and lower toxicity to mammals and birds [5] . They definitely enter the aquatic environment by methods such as harvest dusting, plantation and forest spraying [6] . Deltamethrin (DM) ((S) α- cyano-3-phenoxybenzyl-(1R)-cis-3-(2.2-dibromovinyl)-2,2 dimethyl cyclopropane carboxylate) is a type II pyrethroid that has found wide acceptability [7] , in farming, forestry, agriculture, against nuisances on animals and vermin on wooden materials and plants around the world.
In most recent couple of decades, DM has generally been used as home and garden insecticides connected broadly for the control of mosquitoes and in the treatment of ectoparasitic illness. Pyrethroids, including DM are practically non-harmful to birds but highly toxic to fish and aquatic invertebrates because they are metabolized and wiped out essentially slower in fish than mammals or fowls [8] . Therefore, the toxicity of DM in fishes is basically ascribed to its high rate of gill absorption because of its lipophilicity and inadequacy in the fish enzyme system to hydrolyze pyrethroids [9] . Biochemical profiles of blood are studied and considered as essential wellbeing markers that give important information about the internal environment of the organism [10] - [12] , and frequently used when fish physiology is connected to analyze and comprehend the toxicological effects of external stressors and harmful substances [13] . Oxidative stress and reactive oxygen species (
ROS
) mediated toxicity have long been considered as the responsible mechanisms for DM-prompted organ injury in mammals [14] and some fish [7] . Thus, oxidative anxiety occurs when
ROS
overpower the cellular defenses, causing damage to proteins, membranes, and
DNA
[15] [16] , and is characterized as an interruption of the pro-antioxidant balance, which leads to potential damage. In all organisms, the main anti-oxidative enzymes that serve to recover
ROS
are superoxide dismutase (SOD), glutathione peroxidase (GPx), catalase (
CAT
) and other low molecular weight scroungers, for example, reduced glutathione (
GSH
) [17] [18] . Therefore, several studies demonstrated that changes in the levels of antioxidant enzyme activities can be used as possible biomarkers in different aquatic beings [19] . Spirulina platensis (SP) is a filamentous microalgae fitting in with the class of cyanobacteria with characteristic photosynthetic capacity [20] which became one of the most commonly used microalgae in aqua feeds as it is a rich wellspring of protein, vitamins, minerals, crucial unsaturated fats, key amino acids and pigments such as carotenoids that have strong antioxidant and anti-inflammatory activities [21] [22] . Recently, SP has gained more attention in fish not only for its growth-promoting and immunomodulatory effects but also for its antioxidant potential [23] . The African catfish, Clarias gariepinus was selected in this study as it is distributed throughout Africa [24] . Such a species is of commercial significance because of its high development rate, high stocking-thickness limits, high buyer worthiness and high imperviousness to poor water quality and oxygen depletion [25] [26] . A few records have demonstrated that Clarias fishery contributes around 17% of the yearly fish production from all fisheries areas [27] . Moreover, the African catfish has been utilized as a part of key investigates and considered as a brilliant model for toxicological studies [28] . The objectives of the present study were three folds: 1) to determine LC50 of DM in C. gariepinus, 2) to investigate the effect of sublethal DM concentrations on blood biochemical parameters in catfish manifested by oxidative stress path ways in different fish tissues and 3) to evaluate the protective role of SP against DM toxicity in C. gariepinus.
2. Materials and Methods
2.1. Chemicals
Deltamethrin was purchased as a commercial product (Butox®) 50 mg/ml from El-Naser Pharmaceutical Co. (Cairo-Egypt). Pure Spirulina platensis powder was obtained from (Herba Force, UK). All biochemical kits (AST, ALT, ALP, creatinine, urea, uric acid, total protein, albumin, MDA, GSH, SOD, CAT and GSH-Px) were purchased from Bio-Diagnostic Co. (Dokki, Cairo, Egypt).
2.2. Fish Maintenance
A total number of 350 alive catfish C. gariepinus (average weight 500 ± 10 g., length 41 ± 3 cm) were obtained from Abassa fish farm, El-Sharkya governorate, Egypt. Fish were transferred to the laboratory in 100 liters well aerated fiberglass tanks. Fish were treated with (0.5% w/v) potassium permanganate solution for 1 min to remove dermal adherents. The fish were acclimatized for 2 weeks in identical glass aquaria measuring (80 × 40 × 40 cm) having 80 liter of dechlorinated aerated tap water under laboratory conditions (at temperature 25˚C ± 2˚C and natural photoperiod 12 h). The water parameters were as follows: pH 7.5 ± 0.3, temperature 28˚C ± 1˚C, dissolved oxygen 6.5 ± 0.4 mg/l, alkalinity 122 mg/l and hardness 152 mg/l CaCO3. Fish were fed on commercial ration at ratio of 3% of b.w./day.
2.3. Experimental Design
2.3.1. Determination 96 h LC50 of Deltamethrin
The half lethal concentration (LC50) of DM was determined with definitive test by the static renewal bioassay method of Litchfield and Wilcoxon [29] . Briefly, five groups each of eight fish were exposed to various concentrations of DM (10.0025, 20.005, 40.01, 80.02 and 160.04 µg/l), plus the control group.
2.3.2. Sublethal Exposures
After acclimation, the fish were divided into seven groups, each group containing 10 fish, and received the following treatments for 30 days: Fish in Group 1 acted as control group. Fish in Group 2 administered saline orally. Fish in Group 3 administered SP (150 mg/kg b.w.) orally. Fish in Group 4 exposed to 5.19 µg/l DM. Fish in Group 5 exposed to 5.19 µg/l DM and administered SP orally (150 mg/kg b.w.). Fish in Group 6 exposed to 12.97 µg/l DM and fish in Group 7 exposed to 12.97 µg/l DM and administered SP orally (150 mg/kg b.w.) as well as three replicates of control and experimental groups. To keep a constant DM concentration, fish were transferred to freshly prepared toxicant solution every 48 h.
2.3.3. Collection of Blood Samples
At the end of the experiment, blood was collected from the caudal vessels of the individual fish after anesthetization with 0.02% benzocaine solution. Blood samples were allowed to clot at room temperature, then centrifuged at 3000 rpm., at 4˚C for 15 minutes and sera were separated for the determination of biochemical parameters.
2.3.4. Biochemical Analysis
Serum AST and ALT were estimated by the method of Reitman and Frankel [30] . ALP was determined using an enzymatic colorimetric method according to Tietz et al. [31] . Creatinine was measured according to Larsen [32] , urea according to Coulombe and Favreau [33] and uric acid according to Whitehead et al. [34] . Serum total protein and albumin were measured according the method of Lowry et al. [35] and Doumas et al. [36] , respectively.
2.3.5. Tissue MDA and Antioxidant Biomarkers
Samples from liver, kidney and gill tissues were homogenized in cold phosphate buffer saline (0.1 M pH 7.4) using a Potter-Elvejhem glass/Teflon homogenizer. Then, this homogenate was filtered and centrifuged at 1600 rpm. at 4˚C for 10 min; the supernatant was stored at −20˚C until analysis. This supernatant (20%) was used for the determination of MDA according to the method of Mihara and Uchiyama [37] . GSH concentration was determined using the method of Beutler et al. [38] . The activity of SOD was determined by the method described by Nishikimi et al. [39] . Determination of CAT activity was according to the method of Aebi [40] . GSH-Px activity was determined according to Paglia and Valentine [41] .
2.3.6. Statistical Analysis
Data were statistically analyzed using analysis of variance, one way “ANOVA”, and Duncan’s multiple range test to evaluate comparison between means at P < 0.05 (SPSS, 2004).
3. Results
3.1. LC50
C. gariepinus were exposed to different concentrations of DM for 96 h. The results revealed that the 96 h-LC50 was 51.89 µg/l (Table 1) and therefore, 1/10 and 1/4 of the median lethal concentrations (5.19 and 12.97 µg/l) were used for chronic toxicity exposure.
![]()
Table 1. Half lethal concentration (LC50)/96 h of DM for C. gariepinus.
Half lethal concentration of DM = Highest conc. ?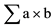 /n. LC50 = 160.04 - 865.22/8 = 51.89 µg/l. Where: a: Constant factor of difference between groups. b: Mean value of dead fish between each two successive groups. n: Number of fish in each group.
/n. LC50 = 160.04 - 865.22/8 = 51.89 µg/l. Where: a: Constant factor of difference between groups. b: Mean value of dead fish between each two successive groups. n: Number of fish in each group.
3.2. Biochemical Parameters
In the present study, C. gariepinus fish exposed to both concentrations of DM showed a significant increase in the level of serum liver enzymes AST, ALT and ALP compared to the control group (Table 2). The combined treatment of DM and SP resulted in significant improvement in AST, ALT and ALP levels. Similarly, serum creatinine, urea and uric acid levels were also significantly elevated with DM toxicity (Table 2). Co-treatment of SP significantly (P ≤ 0.05) reversed serum renal parameters to nearly normal values. Contrary to these results, a marked reduction in total protein and albumin levels were observed in the DM-treated groups compared to the control fish (Table 2). Simultaneous treatment of SP significantly (P ≤ 0.05) increased these levels compared to that of DM-intoxicated group. No significant difference in the other biochemical parameters was observed between fish supplemented SP and the control group.
![]()
Table 2. Serum biochemical parameters in control and experimental groups.
Data are represented as means ± SE (n = 10). Values with different superscript letter in the same row for each parameter are significantly different (P < 0.05).
3.3. Tissue MDA and Antioxidant Biomarkers
MDA levels were significantly increased in the tissues namely, liver, kidney and gill of DM-exposed fish when compared to non-treated ones. Treatment with SP significantly (P ≤ 0.05) reduced MDA level in comparison to DM-exposed fish. SP alone had no significant effects in the liver, kidney, and gill MDA levels (Figure 1). Hepatic, renal and gill tissue of GSH level showed a significant depletion in the groups administered DM compared to the control group. Combination of DM and SP significantly (P ≤ 0.05) enhanced tissue GSH level compared to the DM-treated fish. There were no significant differences between enzyme activity of the group that was administered SP alone and the control group (Figure 2). The activity of SOD, CAT and GSH-Px were significantly decreased in the liver, kidney and gill tissue samples of the groups treated only with DM. On the other hand, Tissues from fish treated with SP showed significant (P ≤ 0.05) improvement in the activity of SOD, CAT and GSH-Px than the DM-treated groups. Fish administrated SP exhibited no significant difference in the tissue SOD, CAT and GSH-Px levels compared to the control group (Figures 3-5).
![]()
Figure 1. Showing liver, kidney and gill MDA levels (nmol/g tissue) in C. gariepinus exposed to 1/10 and 1/4 LC50 of DM and treated with SP (150 mg/kg b.w.) for 30 days. Group 1, control, Group 2, orally administered saline, Group 3, orally administered SP (150 mg/kg b.w.), Group 4, exposed to 5.19 µg/l DM, Group 5, exposed to 5.19 µg/l DM and orally administered SP (150 mg/kg b.w.), Group 6, exposed to 12.97 µg/l DM, Group 7, exposed to 12.97 µg/l DM and orally administered SP (150 mg/kg b.w.).
![]()
Figure 2. Showing liver, kidney and gill GSH levels (mg/g tissue) in C. gariepinus exposed to 1/10 and 1/4 LC50 of DM and treated with SP (150 mg/kg b.w.) for 30 days. Group 1, control, Group 2, orally administered saline, Group 3, orally administered SP (150 mg/kg b.w.), Group 4, exposed to 5.19 µg/l DM, Group 5, exposed to 5.19 µg/l DM and orally administered SP (150 mg/kg b.w.), Group 6, exposed to 12.97 µg/l DM, Group 7, exposed to 12.97 µg/l DM and orally administered SP (150 mg/kg b.w.).
![]()
Figure 3. Showing liver, kidney and gill SOD levels (µg/g tissue) in C. gariepinus exposed to 1/10 and 1/4 LC50 of DM and treated with SP (150 mg/kg b.w.) for 30 days. Group 1, control, Group 2, orally administered saline, Group 3, orally administered SP (150 mg/kg b.w.), Group 4, exposed to 5.19 µg/l DM, Group 5, exposed to 5.19 µg/l DM and orally administered SP (150 mg/kg b.w.), Group 6, exposed to 12.97 µg/l DM, Group 7, exposed to 12.97 µg/l DM and orally administered SP (150 mg/kg b.w.).
![]()
Figure 4. Showing liver, kidney and gill CAT levels (µg/g tissue) in C. gariepinus exposed to 1/10 and 1/4 LC50 of DM and treated with SP (150 mg/kg b.w.) for 30 days. Group 1, control, Group 2, orally administered saline, Group 3, orally administered SP (150 mg/kg b.w.), Group 4, exposed to 5.19 µg/l DM, Group 5, exposed to 5.19 µg/l DM and orally administered SP (150 mg/kg b.w.), Group 6, exposed to 12.97 µg/l DM, Group 7, exposed to 12.97 µg/l DM and orally administered SP (150 mg/kg b.w.).
![]()
Figure 5. Showing liver, kidney and gill GSH-Px level (nmol/g tissue) in C. gariepinus exposed to 1/10 and 1/4 LC50 of DM and treated with SP (150 mg/kg b.w.) for 30 days. Group 1, control, Group 2, orally administered saline, Group 3, orally administered SP (150 mg/kg b.w.), Group 4, exposed to 5.19 µg/l DM, Group 5, exposed to 5.19 µg/l DM and orally administered SP (150 mg/kg b.w.), Group 6, exposed to 12.97 µg/l DM, Group 7, exposed to 12.97 µg/l DM and orally administered SP (150 mg/kg b.w.).
4. Discussion
The96 h LC50 value of DM in C. gariepinus was found as 51.89µg/l in the present study; hence, DM is considered to be highly toxic to fish. The current results are relevant with its potential risk and in good agreement with Datta and Kaviraj [42] who reported 96 h LC50 value of DM on catfish as 40.01 µg/l. The determined 96 h LC50 of DM was 0.38 mg/l for Indian major carp, Labeo rohita (L. rohita) [43] , 0.0142 mg/l for the freshwater fish, Puntius chrysopterus (P. chrysopterus) [44] , 0.7 µg/l for rainbow trout, Oncorhynchus mykiss (O. mykiss) [2] , 14.6 µg/l for Oreochromis niloticus (O. niloticus) (monosex type) [45] . Studying the biochemical parameters of exposed fish, may be among the more sensitive indicators of early changes due to the hazardous exposure to insecticides [46] . The tested catfish exposed to DM pesticide showed a significantly (P < 0.05) increased serum AST,
ALT
and ALP activities. The correlation between elevation of hepatic enzymes with liver damage and dysfunction was reported in many studies [23] [45] [47] [48] . Therefore, measurement of transaminases and phosphatase activities in blood plasma of fish can be used as indicator for pesticide toxicity [49] . The increase in renal parameters could be explained by the changes in kidney tissue through the shrinkage of glomeruli and the breakdown of Bowmanʼs capsules of the exposed fish [23] [45] - [47] . Also, the elevation in creatinine level may result from oxidative damage [50] . Proteins are the most essential macromolecules in living beings, which play a vital role in architecture and physiology of the cell and in cellular metabolism [46] . The reduction of total serum protein and albumin at both concentrations of DM could be attributed to adjustment of the fish to its new environmental circumstances as a result of stress response [51] . Similar results were recorded after exposed C. gariepinus to diazinon and DM, respectively [46] [47] . In contrary, Velíšek et al. [52] estimated a significant increase in total protein and albumin in rainbow trout. The findings of this study suggested that SP provided a marked normalization of the serum biochemical parameters. Oxidative stress in aquatic organisms, particularly fish, has a great importance for environmental and aquatic toxicology. Because oxidative stress is induced by many chemicals, including some pesticides, these pollutants may stimulate
ROS
and alteration in antioxidant systems [53] . It is well documented that DM may prompt oxidative stress [7] [54] . In the current study, exposure of the fish to DM at both concentrations induced an increase in MDA level of the hepatic, renal and gill tissues. Previously, DM was reported to prompt an increase in
MDA
levels in Cyprinus carpio (C. carpio) [3] , O. niloticus [23] and C. gariepinus [47] . The increase in
MDA
level following DM exposure may be attributed to an excessive production of
ROS
[55] . However, the simultaneous treatment with SP decreased the levels of
MDA
in the different tissues of DM-treated fish, which may be due to the antioxidant active constituents found in SP such as C-phy- cocyanins, β-carotene, minerals, vitamins, proteins, carbohydrates and lipids [56] . Chronic exposure to DM decreased hepatic, renal and gill non-enzymatic antioxidant,
GSH
levels compared to the control group. Previous investigations have reported the reduction of
GSH
by pesticides such as DM [23] and endosulfan [57] . The decrease in
GSH
level in the tissues of exposed fish may be due to its exploitation to encounter the prevalent oxidative stress under the influence of
ROS
generated from DM oxidative stress. Also, the depletion in
GSH
levels as an adaptive response to the increment in LPO levels, demonstrating severe oxidative stress [58] . As it is known that the antioxidant enzymes
CAT
, SOD and
GSH
-Px are the first line of defense against oxidative stress which convert superoxide anions (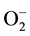 ) into H2O2 and then into H2O and O2 [53] [59] . This view was in agreement with Abdelkhalek et al. [23] who recorded significant decrease in SOD,
CAT
and
GSH
-Px levels in liver, kidney and gill tissues of tilapia fish upon exposure to DM and Hamed [60] who documented marked depletion in the hepatic SOD and
CAT
levels of catfish upon exposure to malathion. This decrease in the SOD activity may be the result of excessive free radical production, such as the superoxide anion and hydrogen peroxide, direct damage of its protein structure by pesticide or a direct action of pesticide on the synthesis of the enzyme [55] . Interestingly, administration of SP increased the activity of
CAT
, SOD and
GSH
-Px; this increase could be attributed to scavenging free radicals or inhibiting lipid peroxidation and superoxide radical formation.
) into H2O2 and then into H2O and O2 [53] [59] . This view was in agreement with Abdelkhalek et al. [23] who recorded significant decrease in SOD,
CAT
and
GSH
-Px levels in liver, kidney and gill tissues of tilapia fish upon exposure to DM and Hamed [60] who documented marked depletion in the hepatic SOD and
CAT
levels of catfish upon exposure to malathion. This decrease in the SOD activity may be the result of excessive free radical production, such as the superoxide anion and hydrogen peroxide, direct damage of its protein structure by pesticide or a direct action of pesticide on the synthesis of the enzyme [55] . Interestingly, administration of SP increased the activity of
CAT
, SOD and
GSH
-Px; this increase could be attributed to scavenging free radicals or inhibiting lipid peroxidation and superoxide radical formation.
5. Conclusion
According to the present study, we can conclude that DM treatment to C. gariepinus caused fluctuations of serum biochemical assays and oxidative damage in different tissues which are sensitive tools for evaluating the effects of DM for monitoring pollution in aquatic medium including fish. On the other hand, SP could be a helpful supplementation for improving the physiological status of fish treated with DM by enhancing their serum biochemical alterations and anti-oxidative capacity.