Synthesis and Characterization of 2,2’-Bipyridyl-5,5’-Dialdehyde Schiff Bases from O,S,N and F-Containing Amines ()
1. Introduction
Design and synthesis of organic chelating agents containing nitrogen and sulfur as donor atoms and their metal complexes is an interesting field of research for their different types of activities. In this case, bi-dentate N,N chelating agent such as 2,2’-bipyridyl has played a vital role in building many mixed-ligand complexes for their desired predictable co-ordination behavior and their electrochemical and photophysical properties [1] -[3] . The 2,2’-bipyridyl and ligands derived from it also extensively used in different areas, such as molecular scaffolding, supramolecular assemblies, catalysis, biochemistry, electrochemistry, ring-opening metathesis polymerization and biochemistry [4] - [8] , biologically photoredox reactions [9] , synthetic, medicinal chemistry, biotechnology [10] and solar cell [11] [12] . It has been observed that Schiff bases formed by condensation of S-alkyl/aryl esters of dithio-carbazaic acid with heterocyclic aldehydes and ketones contain both nitrogen and sulfur that are eligible to form stable complexes with a wide variety of metal ions, some of which have found to show interesting properties [13] - [15] . In view of the importance of Schiff base derived from 2,2’-bipyridyl-5,5’dicarbaldehyde and O,S,N and F-containing amines in different fields, we report here synthesis and characterization of several new Schiff bases from 2,2’-bipyridyl-5,5’-dialdehyde and some sulfur, nitrogen and fluorine containing amines.
2. General Method and Procedures
HPLC grade solvents were used in all the reactions. The conventional method of synthesis of the Schiff bases involves refluxing the reaction mixture containing the dialdehydes and amines for 1 hour followed by filtration of the solid products using suction filtration. In all the reactions, 2 - 3 drops of conc. sulfuric acid were used. The solid product that had formed was filtered off using suction filtration. All the NMR data were recorded on a 400 MHz Varian NMR Spectrometer. Mass Spectra were obtained on a Varian LC-MS with ESI.
3. Synthesis
Eight new Schiff Bases from 2,2’-bipyridyl-5,5’dicarbaldehyde with sulfur containing amines, such as: S-me- thyldithiocarbazate (SMDTC, 1), Thiosemicarbazate (2), S-benzyldithiocarbazate (SBDTC, 3), Thiocarbazate (4), 2-(Methylthio)aniline (5), Pentafluorophenylhydrazine (6), 4-(Trifluoromethyl)phenylhydrazine (7), and 4,4-Di-methyl-3-thiosemicarbazide (8), have been synthesized.
3.1. Synthesis of 2,2’-Bipyridyl-5,5’dicarboxaldehyde 3 from 2,2’-Bipyridine-5,5’-dimethyl
2,2’-bipyridyl-5,5’dicarboxaldehyde was synthesized from 2,2’-bipyridine-5,5’dimethyl following literature procedure [16] . The first step was carried out under nitrogen conditions to produce 5,5’-bis(methylbromo)- 2,2’-bipyridine by bromination following a previously reported procedure [16] . Proton and C-13 NMR data were consistent with the literature value. The yield was 35% (Scheme 1).
Then dibromo compound 2 was hydrolyzed in 50% acetic acid following a previously reported procedure [17] . The pure white precipitate of the product 3 was collected after setting pH at 10 (Y = 75%).
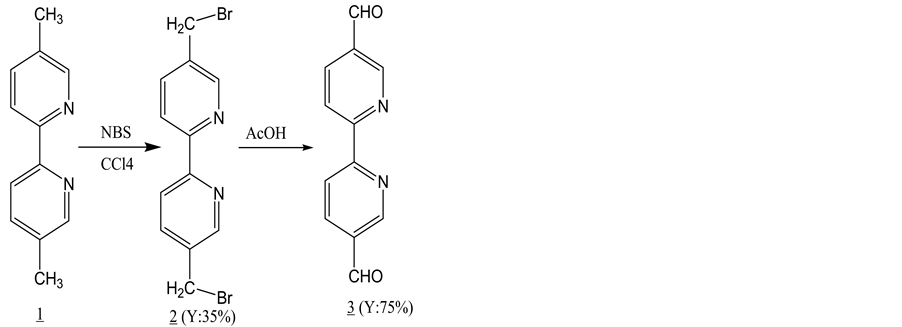
Scheme 1. Synthesis of 2,2’-bipyridyl-5,5-dialdehyde.
3.2. Synthesis of Schiff Base 4 with S-Methyldithiocarbazate (SMDTC) (Scheme 2)
SMDTC was prepared following a literature procedure [18] . 3 equivalent of SMDTC was added to a solution of one equivalent of 2,2’-bipyridine-5,5’dicarboxaldehyde in 25 ml of methanol containing 2 - 3 drops of conc. sulfuric acid. The solution was refluxed for 1 hour and then allowed to cool to room temperature. The solid product formed was filtered off and washed with methanol and dried under vacuum. The isolated yield of the product 4 was 75%. IR, ν (cm−1): 3113 (NH), 2966 and 2859 (CH aromatic and aliphatic), 1592 (C=N), 1515, 1470 (C=C, aromatic), 1592 (C=N), 1050 (C=S). 1H-NMR (DMSO-d6, ppm): δH = 13.50 (s, 2NH), 8.30 (d, 2H), 8.50 (d, 2H), 8.35 (s, 2H), 9.00 (s, 2H), 2.55 (s, 6H). 13C-NMR (DMSO-d6, ppm): δC = 199, 156, 149, 143, 135, 130, 121, 17. LC-MS (m/z): 420 (M+), 421 (M+H+, 100%). Proton and C-13 NMR data indicates that the compound has a two-fold symmetry.
3.3. Synthesis of the Schiff Bases 5 with S-Benzyldithiocarbazate (SBDTC) (Scheme 3)
SBDTC was prepared following previously published procedure [18] . 3 equivalent of SBDTC was added to a solution of 1 equivalent 2,2’-bipyridyl-5,5’dicarboxaldehyde in 30 ml of methanol containing 2 - 3 drops of con. sulfuric acid. The solution was refluxed for 1 hour and then allowed to cool to room temperature. The solid product formed was filtered off and washed with methanol and dried under vacuum (Y = 85%). IR, ν (cm−1): 3200 (NH), 3060, 2916 (CH aromatic and aliphatic), 1574 (C=N), 1590, 1470 (C=C, aromatic), 1050 (C=S). 1H-NMR (DMSO-d6, ppm): δH = 13.50 (s, 2NH), 8.30 (d, 2H), 8.50 (d, 2H), 8.05 (s, 2H), 8.9 (s, 2H), 7.3 (m, 3H),4.55 (s, 2H). 13C-NMR (DMSO-d6, ppm): δC = 197, 156, 149, 143, 137, 135, 129, 128, 127,121, 52. LC-MS (m/z): 573 (M + H+), 572 (M+H+) (100%).
3.4. Synthesis of Schiff Base 6 with Thiosemicarbazate (Scheme 4)
3 equivalent of thiosemicarbazate was added to a solution of 1 equivalent of 2,2’-bipyridyl-5,5’dicarboxaldehyde in 30 ml of MeOH. The solution was refluxed for 1 hour and then allowed to cool to room temperature. The solid product formed was filtered off and washed with methanol and dried under vacuum (Y = 75%). The following spectral data confirmed the formation of the desired Schiff base 6. IR, ν (cm−1): 3433, 3372 (NH), 3279 (N-H), 3168, 2900 (CH aromatic), 1599 (C=N1530, 1600 (C=C, aromatic), 1097 (C=S). 1H-NMR (DMSO-d6, ppm): δH = 11.67 (s, 2NH), 8.20 (d, 2H), 8.30 (d, 2H), 8.05 (s, 2H), 8.9 (s, 2H). 13C-NMR (DMSO-d6, ppm): δC = 176, 155 , 149, 139, 135, 131, 130. LC-MS (m/z): 388 (M+), 389 (M+H+) (100%).
3.5. Synthesis of Schiff Base 7 with Thiocarbazate (Scheme 5)
3 equivalent of thiocarbazate was added to a solution of 1 equivalent of 2,2’-bipyridine-5,5’dicarboxaldehyde in 30 ml of methanol. The solution was refluxed for 1 hour and then allowed to cool to room temperature. The solid product formed was filtered off and washed with methanol and dried under vacuum. IR, ν (cm−1): 3433, 3372 (NH2), 3279 (N-H), 3168, 2900 (CH aromatic), 1599 (C=N), 1530, 1600 (C=C, aromatic), 1097 (C=S). 1H-NMR (DMSO-d6, ppm): δH 11.71 (s, 2 NH), 10.01 (s, 2NH), 8.40 (d, 2H, aromatic), 8.60 (d, 2H, aromatic), 8.05 (s, 2H, aromatic), 9.05 (s, 2H, enamine). 13C-NMR (DMSO-d6, ppm): δC = 199, 146, 139, 129, 127, 126, 119. LC-MS (m/z): 358(M+), 359 (M+H+) (100%).
3.6. Synthesis of Schiff Base 8 with 2(Methylthio)-Marcaptoaniline (Scheme 6)
4 equivalent of 2(methylthio)-marcaptoaniline was added to a solution of one equivalent of 2,2’-bipyridyl- 5,5’dicarboxaldehyde in 30 ml of methanol containing 2 - 3 drops of conc. sulfuric acid. The solution was refluxed for 1 hour and then allowed to cool to room temperature. The solid product formed was filtered off and washed with methanol and dried under vacuum (Y = 55%). IR, ν (cm−1): 3168, 2900 (CH aromatic), 1599 (C=N), 1530, 1600 (C=C, aromatic). 1H-NMR (DMSO-d6, ppm): δH 8.51 (d, 2H), 8.57 (d, 2H), 8.66 (s, 2H), 9.22 (s, 2H, enamine). 13C-NMR (DMSO-d6, ppm): δC = 157, 156, 151, 148, 136, 135, 132, 127, 125, 124, 121, 117, 14. LC-MS (m/z): 454(M+), 455 (M+H+) (100%).
3.7. Synthesis of the Schiff Base 9 with 4-Methyl-Semicarbazate (Scheme 7)
3 equivalent of 4-methyl-semicarbazate was added to a solution of one equivalent of 2,2’-bipyridine-
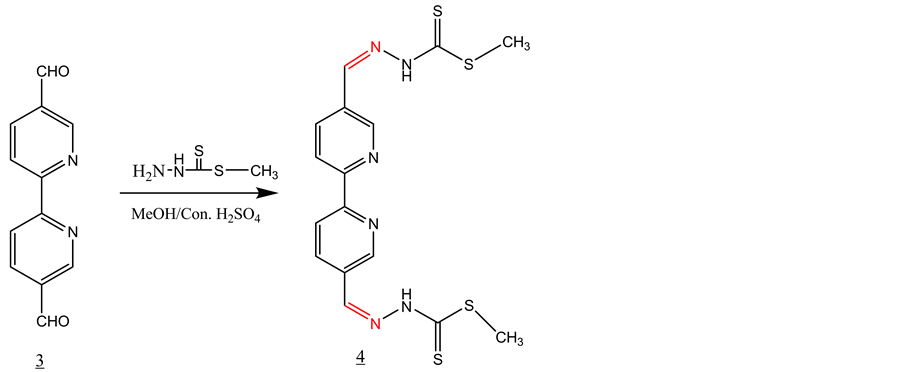
Scheme 2. Synthesis of Schiff base 4 with S-methyldithiocarbazate (SMDTC).
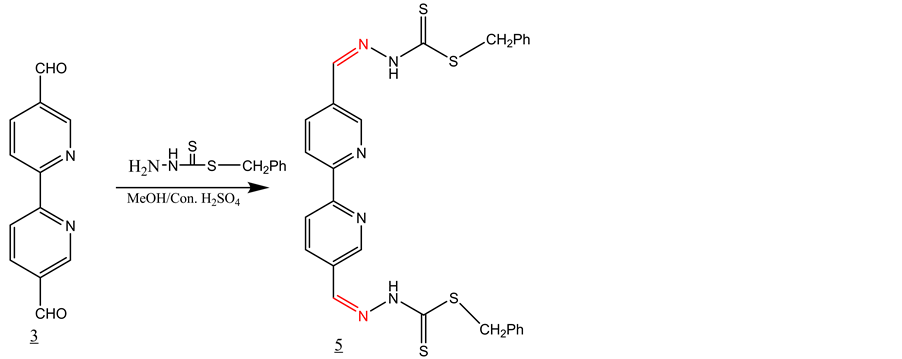
Scheme 3. Synthesis of the Schiff bases 5 with S-benzyldithiocar- bazate (SBDTC).
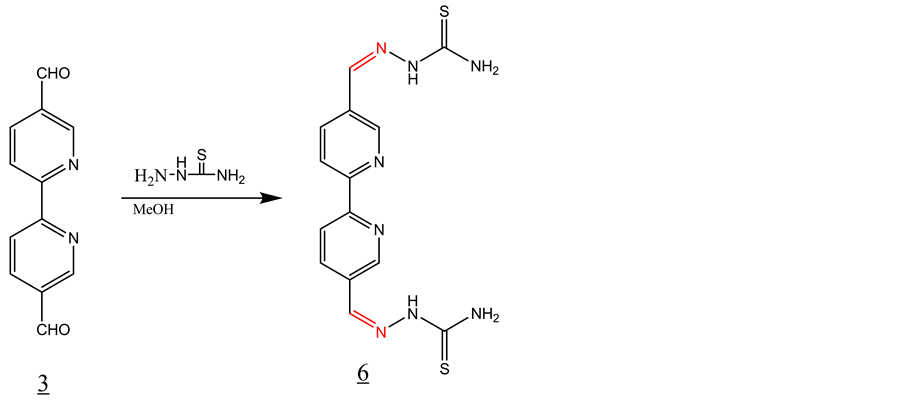
Scheme 4. Synthesis of Schiff base 6 with thiosemicarbazate.
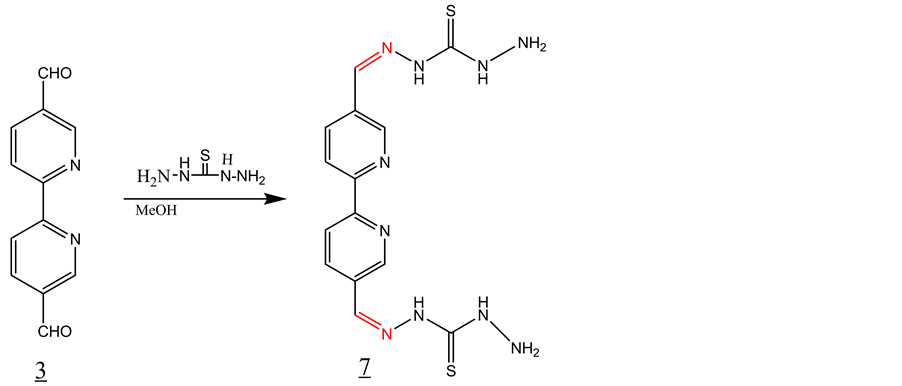
Scheme 5. Synthesis of Schiff base 7 with thiocarbazate.
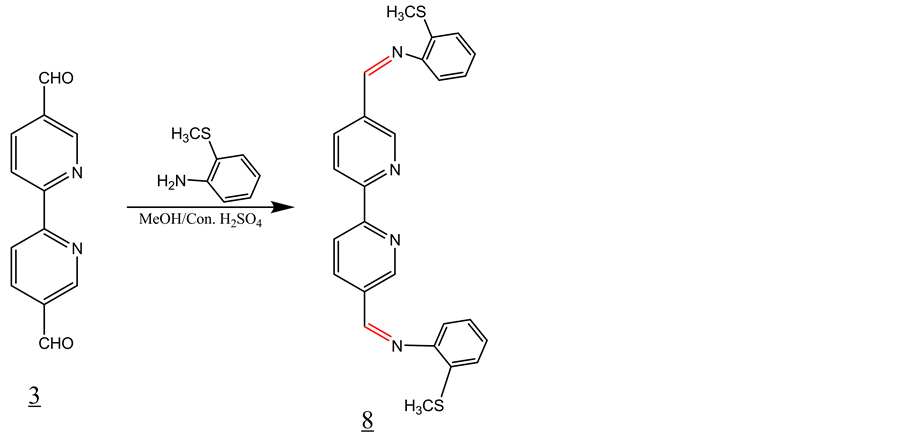
Scheme 6. Synthesis of Schiff base 8 with 2(methylthio)-marcaptoa- niline.
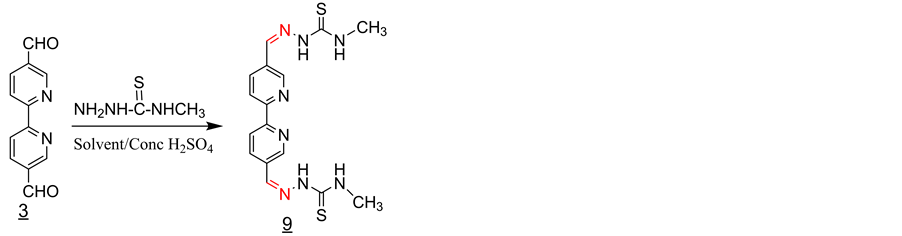
Scheme 7. Synthesis of the Schiff base 9 with 4-methyl-semicarba- zate.
5,5’dicarboxaldehyde in 25 ml of methanol containing 2 - 3 drops of con. sulfuric acid. The solution was refluxed for 1 hour and then allowed to cool to room temperature. The solid product formed was filtered off and washed with methanol and dried under vacuum (Y = 60%). IR, ν (cm−1): 3373, 3156 (2N-H), 3001 (CH aromatic and Aliphatic CH), 1552 (C=N) 1530, 1523 (C=C, aromatic), 1097 (C=S). 1H-NMR (DMSO-d6, ppm): δH = 11.85 (s, 2NH), 9.01 (s, 2NH), 8.75 (d, 2H), 8.40 (s, 2H), 8.05 (s, 2H), 3.00 (s, 6H). 13C-NMR (DMSO-d6, ppm): δC = 178, 155, 149, 138, 135, 131, 130, 31. LC-MS (m/z): 414 (M+), 415 (M+H+) (100%).
3.8. Synthesis of the Schiff Base 10 with Pentaflurophenylhydrazine (Scheme 8)
3 equivalent of pentaflurophenylhydrazine was added to a solution of one equivalent of 2,2’-bipyridyl- 5,5’dicarboxaldehyde in 20 ml of methanol containing 2 - 3 drops of conc. sulfuric acid. The solution was refluxed for 1 hour and then allowed to cool to room temperature. The solid product formed was filtered off and washed with methanol and dried under vacuum (Y = 70%). IR, ν (cm−1): 3433, 3372 (NH2), 3279 (N-H), 3168, 2900 (CH aromatic), 1599 (C=N1530, 1600 (C=C, aromatic), 1097 (C=S). 1H-NMR (DMSO-d6, ppm): δH = 10.70 (s, 2NH), 8.9 (s, 2H), 8.15 (d, 2H), 8.50 (d, 2H), 8.10 (s, 2H). 13C-NMR (DMSO-d6, ppm): δC = 155, 148, 139, 134, 131, 121. LC-MS (m/z): 544(M+), 545 (M+H+) (100%).
3.9. Synthesis of the Schiff Base 11 with 4-(Trifluromethyl) Phenylhydrazine (Scheme 9)
3 equivalent of 4-(trifluromethyl) phenylhydrazine was added to a solution of one equivalent of 2,2’-bipyri- dine-5,5’dicarboxaldehyde in 25 ml of methanol containing 2 - 3 drops of con. sulfuric acid. The solution was refluxed for 1 hour and then allowed to cool to room temperature. The solid product formed was filtered off and washed with methanol and dried under vacuum (Y = 55%). IR, ν (cm−1): 3239 (N-H), 2980 (CH aromatic), 1584 (C=N1530, 1615 (C=C, aromatic), 1092 (C=S). 1H-NMR (DMSO-d6, ppm): δH = 11.10 (s, 2NH), 8.40 (d, 2H), 8.30 (d, 2H), 8.95 (s, 2H), 8.05 (s, 2H), 7.30 (d, 2H), 7.60 (d, 2H). 13C-NMR (DMSO-d6, ppm): δC = 155, 148, 136, 133, 131, 127, 126, 124, 121, 119, 112, 49. LC-MS (m/z): 528(M+), 529 (M+H+) (100%).
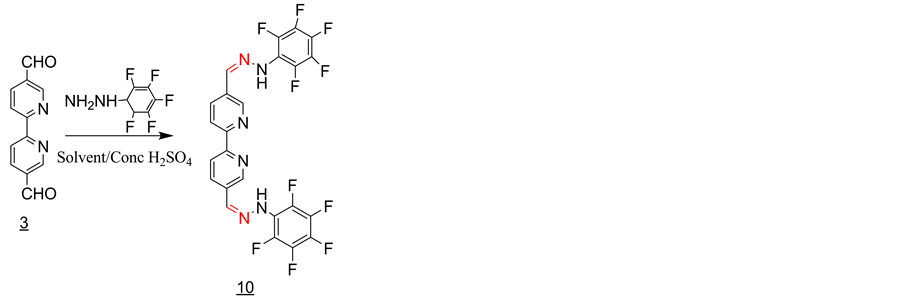
Scheme 8. Synthesis of the Schiff base 10 with pentaflurophenylhydrazine.
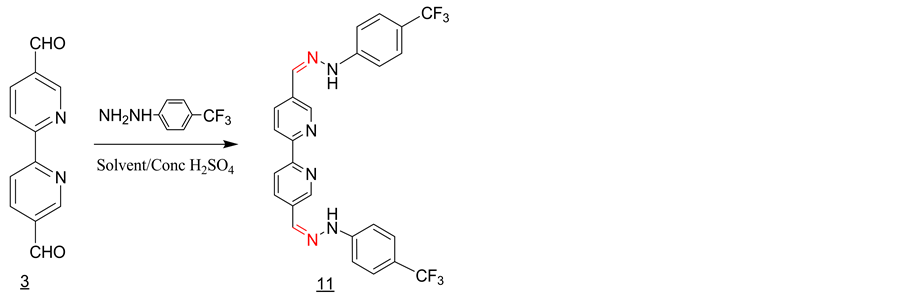
Scheme 9. Synthesis of the Schiff base 11 with 4-(trif- luromethyl) phenylhydrazine.
4. Conclusion
Eight new Schiff bases of 2,2’-bipyridyl-5,5’dialdehyde with O,S,N and F containing amines have been successfully synthesized. Conc. sulfuric addition has been found to significantly enhance the yields of the products. In some cases, sulfuric acid was not added to avoid salt formation with nitrogen and amine group. However, it was observed that the yield increased significantly when the reaction was carried out under mild aicidic conditions. This is due to the fact that protonation of the carbonyl group (C=O) enhances the nucleophilic attack -NH2 group of the amine.
Acknowledgements
We thank the Department of Chemistry at Tennessee State University for providing the necessary support to carry out the research. We also thank the Department of Education, Title III funds for providing instrumental support.
NOTES
*Corresponding author.