1. Introduction
Takotsubo cardiomyopathy (TTC) is a unique disease entity, with a characteristic reversible ventricular dysfunction, making its timely diagnosis quite often a daunting proposition. The ventricular dysfunction associated with this usual entity is often but not always characterized by apical akinesis, and basal hyperkinesis, in a distribution which does not correlate, with a specific, unique, epicardial coronary artery blood supply [1]. Here we discuss a case of Takotsubo cardiomyopathy with extremely rapid resolution, in just two days, which we believe, is being reported for the first time. We review briefly the essential features of this disease including epidemiology, pathogenesis, clinical presentation, treatment, and prognosis.
2. Case Report
A 62 year old lady, with past medical history of hypertension, and chronic obstructive pulmonary disease was admitted with sudden shortness of breath, and chest pain. A few days before this episode, her youngest daughter had died in a traffic accident. Just prior to admission, she experienced sudden and severe shortness of breath at night, along with unrelenting chest pain located in central lower chest area, with no radiation. The chest pain resolved within half an hour, but the shortness of breath persisted, and rapidly worsened.
Upon presentation to the emergency room (ER), her physical exam revealed a blood pressure of 119/87, pulse rate of 84/min, oxygen saturation of 86% on room air, and body temperature of 97.1˚F. There were no murmurs, rubs or gallops, no crackles on chest auscultation, no jugular venous distension, and no pedal edema. She had a mild elevation in her cardiac enzymes (Troponin = 0.05, CK-MB = 1.7, CK = 97), and her Brain-Natriuretic Peptide (BNP) level was 1400. Her laboratory work-up was otherwise within normal limits. Her EKG showed nonspecific ST-T changes, and QT prolongation, with a corrected QT interval (QTc) of 505 ms, and her chest X-Ray showed hyperexpanded lung fields, with flattened diaphragms.
Given her low oxygen saturations, and her shortness of breath, she was initially started on BIPAP (Bi-level positive airway pressure) and was given furosemide, aspirin, heparin infusion, and statin, with the most likely diagnosis being: heart failure secondary to Non-ST Elevation Myocardial Infarction (NSTEMI).
A Transthoracic Echocardiogram (TTE), was performed the next day, and showed marked left ventricular systolic dysfunction (ejection fraction of 15% - 20%), severe apical hypokinesis, basal hyperkinesis, moderately reduced right ventricular function, and mild mitral regurgitation (Figure 1). Left heart catheterization revealed normal coronary arteries, with no obstructive disease.
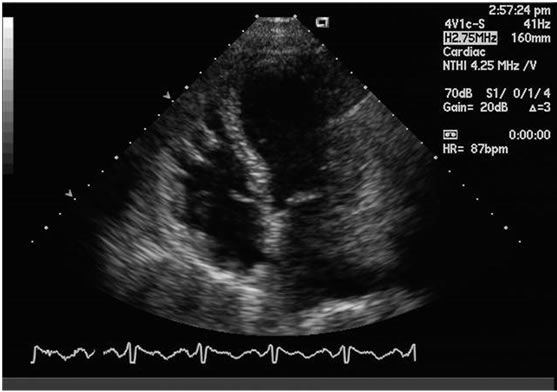
Figure 1. Characteristic apical ballooningin systole with markedly reduced left ventricular ejection fraction.
A repeat TTE was obtained, after two days, following a dramatic resolution of patient’s symptoms. The latter revealed a normal systolic function with an ejection fraction of 55% - 65% with absence of apical hypokinesis (Figure 2). The moderately reduced right ventricular function, and the mild mitral regurgitation, seen previously, had completely resolved, at the time, the second echocardiogram was done.
Interestingly, the nonspecific ST-T changes, and prolonged QTc on admission resolved as well, over a time period of two days.
3. Discussion
Stress-induced cardiomyopathy, also called apical ballooning syndrome, is generally characterized by transient systolic dysfunction of the apical, and/or mid segments of the left ventricle (LV), that mimics myocardial infarction, in the absence of significant coronary artery disease [1].
Typically, the contractile function of the mid, and apical segments, of the LV is depressed with compensatory
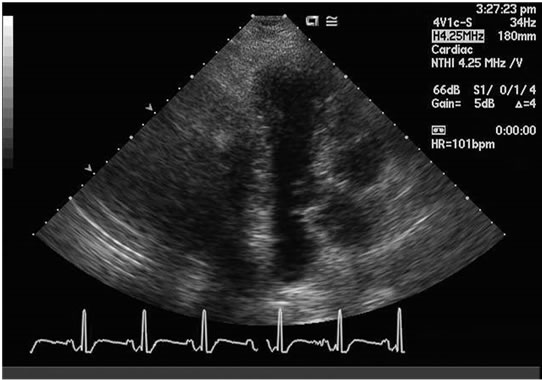
Figure 2. Resolution of the ballooning on the second day with normal left ventricular ejection fraction.
hyperkinesis of the basal walls, producing “ballooning of the apex, during systole. A different type of transient cardiomyopathy, with mid-ventricular akinesis, has also been described (mid-ventricular ballooning syndrome). In a description, of six cases of mid-ventricular ballooning, there was mild ventricular dysfunction (ejection fraction 40%), and shorter recovery times (1 - 7 days) [2]. However, in our case there was typical apical ballooning, with no mid-ventricular involvement, and a significant reduction in left ventricular ejection fraction.
Stress-induced cardiomyopathy, is much more common in women than men. In a review of ten prospective series, women accounted for 80% - 100% of cases, with a mean age of onset, of 61 - 76 years [3]. In a prospective registry of 3,265 patients,frequency of transient cardiomyopathy was 1.2% in troponin positive acute coronary syndrome (ACS) patients [4]. The onset of stress-induced cardiomyopathy, is frequently, but not always triggered by an acute medical illness, or by intense emotional or physical stress [5]. Postulated mechanisms include: catecholamine excess, coronary artery spasm, and microvascular dysfunction among others, which leads to myocardial stunning. Direct catecholamine toxicity has been implicated as well [6].
The clinical presentation, of stress-induced cardiomyopathy, is similar to that of an acute myocardial infarction (MI) [7]. The most common presenting symptom is acute sub sternal chest pain, but some patients present with dyspnea, syncope, shock, or electrocardiographic (EKG) abnormalities [8].
The EKG often reveals ST elevation (mostly precordial) during the acute phase, followed by T wave inversion, QT prolongation, and sometimes deep Q waves during the subacute phase [9,10]. In a comparison study of takotsubo cardiomyopathy and acute myocardial infarction (MI) 34% to 56% of takotsubo patients showed ST elevation in anterior leads [9,10], with absence of reciprocal changes, prolongation of QTc interval, and deep T wave inversion [9,10]. Cardiac biomarkers are usually mildly elevated [10]. Another unique aspect which has come to light is occasional involvement of the right ventricle (RV). In a series of 34 patients, with stress induced cardiomyopathy, it was found that 26% had RV motion abnormalities [11]. In our case, non specific ST-T wave changes, QTc prolongation, and moderate right ventricular dysfunction, were present on admission, and resolved over two days, along with resolution of left ven -tricular dysfunction.
Despite the severity of the acute illness, stress-induced cardiomyopathy is a transient disorder, managed with supportive therapy. Conservative medical treatment with beta-blockers when possible has been thought to be associated with lower mortality during the acute phase [12]. ACE inhibitors, and diuretics, are used as required with volume overload and aspirin in setting of coexisting coronary atherosclerosis. Resolution of the physical or emotional stress, usually results in rapid resolution of symptoms [13], and EKG changes.
In-hospital mortality rates have been reported by Gianni et al., as 1.1%, along with a recurrence rate of 3.5% [14]. In a prospective study of 136 patients with stress cardiomyopathy the mortality rate was 2% [15]. However, in another retrospective study of 100 patients, with a mean follow up of 4.4 ± 4.6 years the recurrence rate was 11.4%, over 4 years after initial presentation [16].
The recovery times, for typical cases with apical ballooning, are highly variable. Patients who survive the acute episode, typically recover normal ventricular function within 1 - 4 weeks, as shown in a study of 30 patients, with takotsubo cardiomyopathy [17]. In this case, the ground for unexpectedly quick resolution, within two days, is difficult to pinpoint with certainty.
In a study of 22 patients, over a 32 month period the mean LVEF increased from 29% at initial presentation to 63% at a mean of 6 days [17]. In another study of 19 patients LVEF increased from a median of 20% at presentation to 60% in 2 - 4 weeks [18]. It should be noted though, that daily echocardiogram has not been performed, prospectively on patients admitted with takotsubo cardiomyopathy, and therefore the exact period of resolution, has not been well elucidated so far.
4. Conclusions
This case is to our knowledge, the first case that highlights the dramatic resolution of takotsubo cardiomyopathy, which occurred within two days of presentation. We believe this to be the most rapid EF normalization, ever published to date, for a case of takotsubo cardiomyopathy with typical apical ballooning pattern [19]. Extensive related search of literature to establish this was done, using ‘takotsubo’ and ‘quick resolution’ as the key words. We emphasize the fact that keen insight of physicians is extremely important, as to not overlook the highly diverse clinical scenarios, and unpredictable patterns of this unique disease. This also warrants the need, for future studies to be done, looking at prognostic variables that could be associated with the duration of left ventricular dysfunction.