Seed Germination in Tomato: A Focus on Interaction between Phytochromes and Gibberellins or Abscisic Acid ()
1. Introduction
Classic factors that modulate seed germination in a wide range of species include the hormones gibberellin (GA) and abscisic acid (ABA) and phytochromes (phys), which are converted from their inactive red (R)-light-absorbing form (Pr) into its active far-red (RF)-light-absorbing form (Pfr) [1] . However, although it is well known that separately GA, ABA and phy modulate seed germination [2] , these factors can strongly interact each other. For example, during seed germination in Arabidopsis, the degradation of PHYTOCHROME INTERACTING FACTOR 3-LIKE5 (PIL5) (also called PIF1) by phy reverses the action of PIL5, reducing ABA levels and increasing GA levels, and therefore, decreasing the DELLA proteins, GA negative signaling components [3] [4] . In addition, the DELLA-dependent accumulation of endogenous ABA levels stimulates the expression of ABI3, an embryonic transcription factor that is necessary to repress germination under FR conditions [5] -[7] .
However, the regulatory loops between light and GA and ABA signaling are still very complex since the intricate molecular and biochemical signaling pathways of these molecules are dependent on species, temperature, and light [2] [8] . For example, it is known that Arabidopsis seeds germinate poorly in darkness, but GA can promote seed germination even in the dark [9] . In barley seeds, the expression of GA3ox2, a GA biosynthetic gene, increased rapidly and continued to increase up to 24-h imbibition in after-ripened grains in both light and dark conditions [10] . Thus, there are an overwhelming number of molecular and biochemical questions about the mechanisms behind seed germination. For example, which hormones and photoreceptors control the germination response, how do they do it, and how do they interact with one another? Are these interactions light dependent? In order to answer many of these questions, conducting a physiological dissection of these tools prior to following the molecular approach can provide answers to many of the above questions. Thus, hormonal and photomorphogenic mutants and hormonal-photomorphogenic double mutants have served as important tools. In the present study, we used GA constitutive response, ABA-deficient or phy-deficient mutants, as well as GAphy or ABA-phy double mutants of tomato to explore the physiology of seed germination.
2. Materials and Methods
Plant Material and Seed Germination Assays
We used seeds of tomato (Solanum lycopersicum) mutants introgressed into the cultivar Micro-Tom (MT) [11] : aurea or au (phy-deficient) [12] , sitiens or sit (ABA-deficient) [13] , and procera or pro (GA constitutive response) [14] which were kindly provided by R. Chetelat (The C.M. Rick Tomato Genetics Resource Center, Davis, USA). We also used au sit and au pro double mutants previously constructed [15] . MT was used as control. Seed germination assays of photomorphogenic, hormonal, and double mutants and MT control were performed by sowing seeds onto two wet filter papers in transparent or black plastic boxes. The experiments were conducted in a growth chamber (25˚C, 16 h photoperiod, 55 μmol photons m−2∙s−1 PAR). For the dark treatment, seeds were counted in a dark room under a dim green safelight. We used three replicates of 50 seeds and calculated the daily (i) (12/12 h for 120 h) percentage of seeds that germinated (Gi%), besides the Gf%, where f is final percentage (after 120 h). The calculation may be expressed using the following formula:
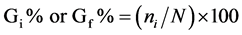
where ni is the number of seeds germinated every 12 h, and N is the number of seeds included in the test, and then Gf% is final percentage (after 120 h) of seeds that germinated.
We used the following formulas to calculate the germination speed index (GSI) and average germination time (AGT):
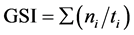
where ti is day i
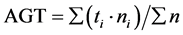
T50 is the time it takes to reach 50% germination. This, in turn, is calculated according to the following formula [16] [17] :
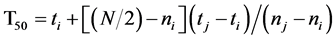
where N is the final number of seeds that germinated and ni and nj are the cumulative number of seeds germinated by adjacent counts at times ti and tj when ni < N/2 < nj.
To observe seed dormancy break as affected by GA in au mutant, seeds were incubated in light and dark conditions during 120 h with 100 µM GA.
Germination was defined as the visible emergence of the radicle through the seed coat [18] [19] .
The data were submitted to statistical analysis by using analysis of variance and Tukey’s test at a 5% significance level.
3. Results and Discussion
In this study, we used phy, ABA, and GA mutants, as well as double mutants displaying both phy and ABA or both phy and GA mutations, to explore the photomorphogenic and hormonal control of seed germination in tomato. As we expected, given the light treatment the ABA-deficient mutant, sit, showed a constant increase of Gi% after 60 h (Figure 1(A)) and a reduction in GSI, AGT, and T50 (Table 1) compared with the MT control. In fact, in
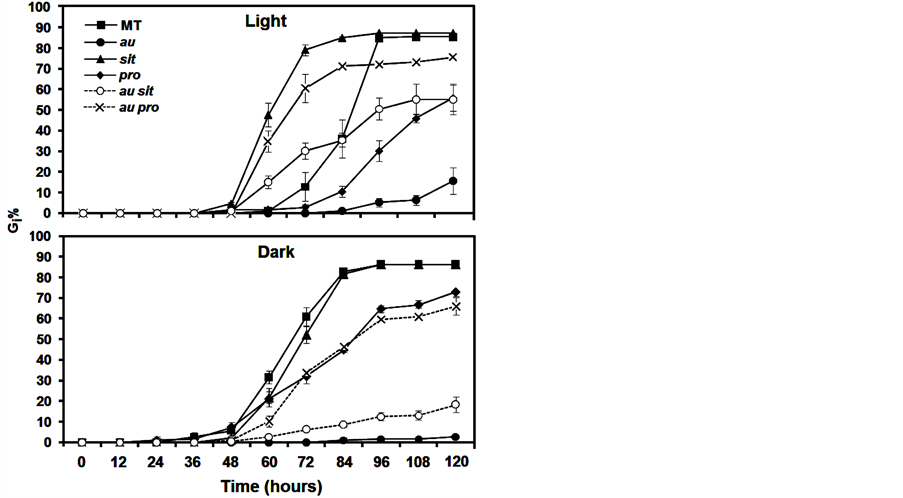
Figure 1. Seed germination percentage at 12 h intervals (i) (Gi%) of photomorphogenic and/or hormonal mutants incubated in light and dark conditions during 120 hours. Data are means ±SE.

Table 1. Germination speed index (GSI), average germination time (AGT), time to reach 50 % germination (T50) and final germination percentage (Gf%) obtained from seeds of photomorphogenic and/or hormonal mutants incubated in light (L) and dark (D) conditions during 120 h.
*In a column, means followed by the same letter are not significantly different by the Tukey test at 5% of probability. Following N/2 (Coolbear et al., 1984; Farooq et al., 2005) [16] [17] , the absent data mean that genotypes did not reach T50.
contrast with wild-type seeds, seeds of sit always readily germinate and even exhibit viviparous germination in overripe fruits [20] . Although the GSI and AGT data show the rapidity with which sit can germinate despite its ABA deficiency, Gf% and Gi% after 120 h did not differ from that of MT (Table 1). Moreover, in the dark condition, Gi% from 48 to 120 h (Figure 1(B)), AGT, T50, and especially GSI (Table 1) did not differ from MT. This finding indicates that ABA deficiency in sit has a complex mechanism that is light dependent during germination and that remains to be explored using molecular and biochemical approaches.
To investigate whether the constitutive GA response conferred by the pro affected germination, Bassel et al. [13] dissected the embryo axes from the endosperm after 4 h of imbibition and verified that the pro germinated the fastest of all the wide types. However, we observed that in both the dark and the light condition in intact seeds, pro reduced Gi% from 60 to 120 h (Figure 1) and also reduced GSI and Gf%. The AGT was similar to that in MT (Table 1), and T50 was increased in the dark. Thus, irrespective of whether constitutive GA response is the mechanism by which pro accelerates germination in dissected seeds, the pro mutation is not fully capable of mediating a rapid breaking of coat dormancy. In other words, although GA profoundly influences seed germination and the pro mutation regulates GA responses within the embryo [13] , the coat modifications for radicle protrusion appear not to depend on the GA constitutive response. Further detailed inspections of seed biochemistry and the anatomy of pro are necessary to explain the delayed germination in this mutant.
One of the more noticeable effects of the au mutation is a severe delay in seed germination [15] [21] due to a deficiency in phytochrome chromophore biosynthesis. We present novel observations relating to this effect regarding Gi% (Figure 1), GSI, and Gf% (Table 1), all of which were reduced in the light and the dark, whereas AGT increased compared to that in MT (Table 1). It is remarkable to note that the severity of the germination delay in the au mutation is intensified in the dark, indicating that although au is phytochrome deficient, either the low phytochrome signaling or the presence of other photoreceptors such as cryptochromes is sufficient to promote germination in this mutant.
Intriguingly, compared with au and pro, the au pro double mutant in the light condition showed an enhanced Gi% from 60 to 120 h (Figure 1(A)), increased GSI (Table 1), and reduced AGT (Table 1). In the dark, this genotype showed a similar GSI and AGT to pro and au, on top of the existing similarity between AGT and pro. One might expect to see an additive effect of reduced germination of au and pro in double mutants, but in the light, the phy deficiency and GA constitutive response improve seed germination. Certainly, these results need to be interpreted carefully, as there is interaction between light and GA at virtually every stage of plant growth [13] [22] . Furthermore, and particularly during seed germination, the mechanisms by which GA is involved in light signaling are quite complex. For example, previous findings in Arabidopsis indicate that phytochromes promote seed germination by lowering the level of DELLA proteins and increasing the level of bioactive GA [3] [4] .
Thus, at least in the light, it is plausible to suggest that in tomato the au mutant shows reduced germination because the phy deficiency does not induce GA activities; however, a hormonal quantification was not carried out in this mutant. So far, although exogenous GA did not recover au germination in the light to MT levels, au seems trigger more sensitivity to GA in the dark (Figure 2), indicating that tomato does not have a linear induction of GA by phy during germination. In fact, although the GA constitutive response of pro recovers au germination in au pro, the delayed germination in pro shows that the GA modulation of seed germination in tomato occurs only under specific conditions or that GA functions are highly redundant. Further evidence is that in the dark, Gi% (Figure 1(B)), GSI, AGT, and Gf% (Table 1) are similar in pro and au pro. In addition, there is an intricate GA signaling pathway in pro or au pro, as pro contains reduced concentrations of GAs despite its constant response to this hormone [23] .
In the light and dark conditions, the intermediary values of Gi% (Figure 1), GSI, and Gf% (Table 1) of au sit compared with au and sit suggest that there is an additive effect of both mutations on germination. However, only in the light condition, AGT of au sit seemed to show an epistatic effect of sit, as sit and au sit are similar (Table 1). Thus, although an additive interaction indicates that light and ABA have independent pathways during tomato germination, AGT of au sit revealed that the mechanisms by which phy and ABA control germination can be dependent on one another. However, data regarding how ABA is part of light signaling are still very scarce in tomato. Majority of the currently available information relates to the separate effects of phy [24] [25] or ABA [26] [27] .
A more detailed analysis of particular aspects of pro, such as the reduced concentrations of GA and constitutive responses to this hormone [23] , will clarify the mechanism for such responses and will improve our knowledge of the role of GA in seed germination in tomato. The results of au pro raise questions about the mechanisms
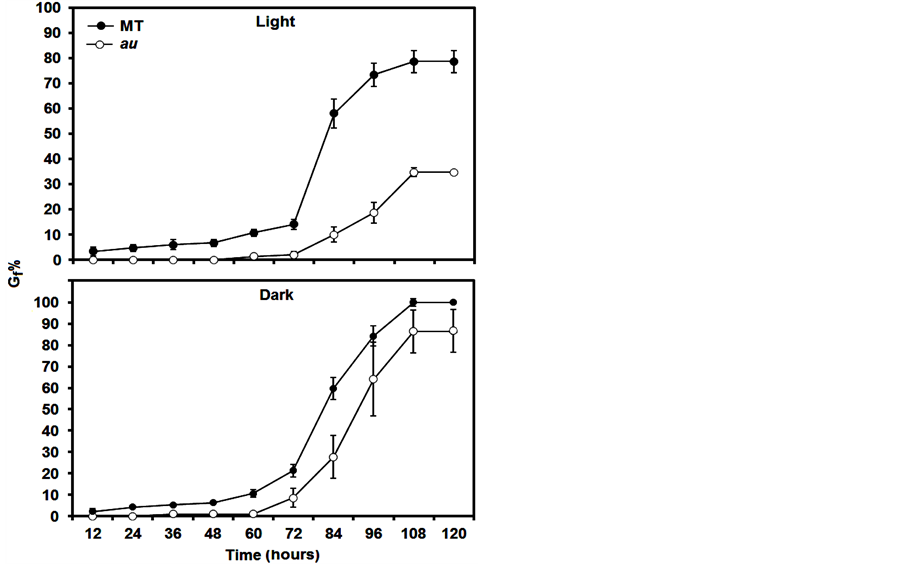
Figure 2. Seed germination percentage at 12 h intervals of au mutant incubated in light and dark conditions during 120 hours. Over time, seeds were treated with 100 µM GA. Note that GA did not induce dormancy break to MT levels, but seems trigger more sensitivity to GA in the dark. Data are means ± SE.
of GA participation in light signaling during germination. For example, which photoreceptors interact with GA, how do they interact, and can they regulate GA levels? These questions may be asked about ABA and other hormonal classes as well, as brassinosteroids, auxin [28] [29] , cytokinin [30] , and ethylene [31] are clearly involved in germination. However, the most important issue of the molecular interpretation of light signaling and hormone interactions during germination remains to be elucidated by future research.
Acknowledgements
The financial support has been provided by FAPESP (grant number 02/00329-8 and fellowship number 03/12416-5) and CNPq (grant number 475494/03-2 and fellowship number 308075/03-0).
Abbreviations
AGT: average germination time GSI: germination speed index GA: gibberellin ABA: abscisic acid
NOTES
*Corresponding author.