Microalbuminuria and Kidney Disease Risk in HIV Patients Taking Combined Antiretroviral Therapy ()
1. Introduction
Aids pandemic is one of the most important causes of human mortality and hospital admission, even today, after more than 30 years of infection [1] . Last epidemiologic data evidenced higher infection dissemination in 1986, with 3.5 millions of new infections [2] . But in 2008 a total of 2.7 million new infections occurred and millions of people died with Aids around the world in 2009. Besides this period evidenced a growing number of persons taking antiretroviral therapy, which permitted an epidemic and mortality control [2] .
Actual antiretroviral therapy can control appropriate viral replication and partially recover Immune System, avoiding diseases progression to AIDS symptoms. But sterilizing cure is not achieved nowadays with current available drugs, specifically due to reservoirs sites in latent cells or probably low levels of viral replication [3] .
Besides an improvement in morbidity and mortality in HIV infected patients, this increased survival revealed others issues as early and late drugs toxicity [4] . Referred as late toxicity we have Metabolic Syndrome, Lipodystrophy, Bone mass loss and Kidney disease [5] [6] .
Kidney Disease (KD) is manifested frequently as urine protein loss or creatinine increase related as complication of HIV infection. High levels of protein loss evidenced worst results, including higher hospital entrance and mortality. Three different studies evidenced high prevalence of microalbuminuria in HIV infected patients, values referred as 19%, 30% and 34%. One of them suggested that symptomatic individuals could have higher risk for microalbuminuria [7] . Since these, antiretroviral therapy has been improving each year and new drugs are available frequently, so kidney damage must be monitored frequently.
Increase in albumin urine excretion, even in microalbuminuria level, is an independent risk for cardiovascular disease (CVD) and Chronic Renal Failure (CRF), with higher mortality in general population and HIV individuals. Occurrences of Kidney diseases in HIV people could be also consequence of drugs toxicity, opportunistic infections, and comorbidities as Systemic Arterial Hypertension, Diabetes Mellitus and Hepatitis C coinfection. Tenofovir inclusion as the first-line antiretroviral therapy was recently recommended by World Health Organization, so risk for kidney diseases may increase in the next years [8] . There is evidence that microalbuminuria could represent an early marker of HIV infection for higher risk of CVD and CRF [9] . Studies in post HAART era evidenced prevalence between 8.7% and 11% in HIV infected patients [10] [11] . The aim of this study is to evaluate kidney damage and renal failure of HIV chronic treated infected patients and correlate with microalbuminuria as a maker, considering risk factors as antiretroviral drugs and comorbidities.
2. Methodology
2.1. Study Setting and Design
Population under analysis consisted in HIV/AIDS patients attended at Fortaleza General Hospital, Ceara, Brazil. This is a reference site to attend HIV infected patients at Ceara and it represents 10% of all diagnosed people in this state. We analyzed three hundred and thirty-six files, and one hundred and forty-nine patients had completed data specifications. Samples of forty-three patients were analyzed to microalbuminuria dosage, in a period from January to December 2013 randomly. This was a retrospective study, with chart files review, and therefore consent term was not demanded.
2.2. Data Collection and Analysis
Sociodemographic data were collected by a closed questionnaire comprising age, sex, use of antiretroviral drugs, occurrence of opportunistic infections, comorbidities, CD4+ T cells count and Viral load. Laboratory assays collected were serum creatinine, serum urea, urine analysis and microalbuminuria in 24 hours. Autoanalyzer manufacturers and clinical laboratories were used to process the samples with regular calibration. Urine volume was collected during 24 hours previous to exam’s process.
Kidney damage was classified by Creatinine Clearance (ClCr) measurement using Crockoft-Gault calculation (Table 1) [12] .
Statistical Package calculated data results analysis for the Social Sciences (SPSS, version 16) for frequency analysis, followed by chi-square and fisher test considering p < 0.05 for significance level.
3. Results
3.1. Demographic Characteristics of Population
One hundred and forty-nine patients were evaluated. Gender distribution comprised 103 (69.1%) males and 46 (39.9%) females. General age mean was 38.5 years, males’ mean 37.8 (±11.6) and females’ mean 40.1 (±10.4) years. Mean time of infection was 65.77 months, with males’ 65.16 (±37.04) and females’ 61.15 (63.35). Mean CD4+ T cells and Viral Load were respectively 600.37 cells/mm3 and 530.59 copies/ml, with 61.7% viral load under 40 copies. There was no statistical difference between groups (Table 2).
Kidney function evaluation evidenced mean creatinine 0.97 ± 1.34 with Creatinine Clearance 110.2 ± 44.6 and Urea 27.76 ± 10.3, without statistical differences between groups (

Table 3).
Table 1. Kidney disease outcomes quality initiative kidney diseases classification.

Table 2. Demographic and immunovirologic characteristcs (n = 149).

Table 3. Serum laboratory analysis of HIV/AIDS patients (n = 149).
3.2. Microalbuminuria and Kidney Dysfunction Evaluation
Analysis of microalbuminuria demonstrated mean microalbuminuria (mg/ml) 32.05 ± 85.25 and microalbuminuria/24h 147.46 ± 820.45. Altered microalbuminuria/24h, defined as above 300, was detected in 8% (n = 12) of total patients. Comparing groups with normal and altered microalbuminuria/24h there was no statistical difference for age, sex, comorbidities, Viral Load and CD4+ T cell count (Table 6), (Graphic 1 and Graphic 2).
A proportion of 6.4% (n = 140) was detected equal or superior to Stage 3 and 6.2% patients had altered microalbuminuria/24h. But, there was no statistical significance correlating microalbuminuria/24h and Stage 3 or above (p = 0.33). Evaluating microalbuminuria mg/dl we detected 11.6% prevalence, but with no correlation to Stage 3 or above (p = 0.31). Urine analysis is evidenced in Table 4.
Evaluating Viral load related to Stage 3 or above, there was no difference between viral load detectable or not (p = 0.17), and presence of microalbuminuria/24h (p = 0.63). There was also no correlation to CD4 below or above 350 cels/mm3 and microalbuminuria/24h (p = 0.6), (Table 5).
In microalbuminuria analysis, mean age was 44.8 (±12.13) years. It was statistic significant has age above 50 years and Stage 3 (p = 0.001), but just 5.7% patients under 50 years and 10% above had microalbuminuria/24h (p = 0.55). Relating comorbidities risk (Diabetes Mellitus plus Systemic Arterial Hypertension) to Kidney Diseases, it was found 55.5% patients in Stage 3 or above with comorbidities compared with 15% with comorbidities in lower stages (P = 0.005) (Table 6). Nevertheless, comorbidities presence was not associated with microalbuminuria (p = 0.08).
Comparing period living with HIV, diagnoses after and before five years was not significant risk for microalbuminuria (p = 0.51). When evaluated drugs used in antiretroviral therapy, Tenofovir (TDF) was associated with Stages 1 (84.3%) and 2 (27.2%), (p = 0.4), protease inhibitors (PI) with Stage 1 (69.7%) and 2 (23.2%), (p = 0.45). Association PI/TDF was presented in 58.3% in Stage 1 and 37.5% Stage 2 (p = 0.79). There was no correlation with microalbuminuria/24h and use of TDF (p = 0.4), PI (p = 1), PI/TDF (p = 0.69), Atazanavir (p = 0.4) or Lopinavir/r (p = 1). Therefore, new drugs seem not to alter this laboratory finding (

Table 7).
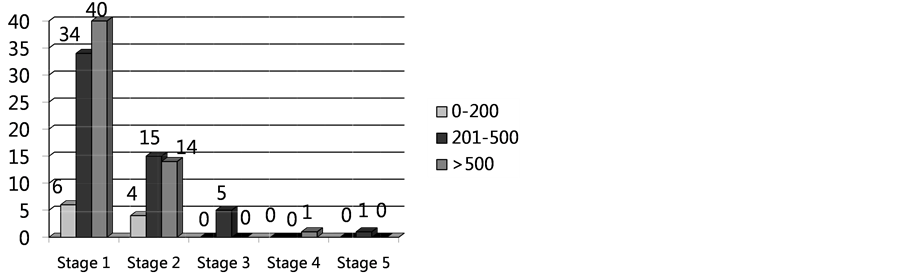
Graphic 1. CD4 count cell correlation to kidney damage classification stages.

Graphic 2. Viral load correlation to kidney damage classification stages.
Table 4. Urine analysis alterations described in HIV patients submitted to microalbuminuria assay.

Table 5. Classification kidney disease in samples from HIV patients and comorbities.

Table 6. Microalbuminuria analysis related to risk factors in HIV/AIDS patients.

Table 7. Comparing antiretroviral drugs and kidney diseases stage classification.
NRTI: nucleoside analogues reverse transcriptase inhibitors; ARV: antiretroviral; TDF: tenofovir, PI: Protease Inhibitors
4. Discussion
In general population, metabolic syndrome has been associated with high rates of albumin urine loss, and this syndrome has been reported recently growing in HIV infected patients. HADIGA et al. (2013) [13] identified microalbuminuria in 26.9% of patients with Metabolic Syndrome, suggesting this condition contribution to kidney damage in HIV. And just one negative sample for microalbuminuria had negative predictive value of 98% and a positive sample 74% of positive predictive value.
Szczech et al. (2007) [7] , evidenced HIV infection as an independent risk factor for microalbuminuria, and suggested it could be a sign of endothelial dysfunction and microvascular disease and not an advanced HIV infection, predicting a future vascular risk. Some factors may contribute to early renal diseases as nephropathy due to HIV, Diabetes Mellitus and Arterial Systemic Hypertension and endothelial dysfunction. This study also associated NNRTI drugs and microalbuminuria but not so strong as HOMA parameters (insulin resistance, Diabetes Mellitus) and CD4 cell count. In the present study we also found association of kidney damage with presence of comorbidities (Diabetes Mellitus as Arterial Systemic Hypertension), but not altered microalbuminuria.
The present study did not find any correlation of microalbuminuria and drug association, even nephrotoxic ones as tenofovir. BAEKKEN, et al. (2008) [9] found the same results, but evidenced a higher prevalence of microalbuminuria in HIV infected patients (8.7%, three to five folds than general population). The period living with HIV infection, serum beta 2 microglobulin and blood systolic pressure were independent risk factor for microalbuminuria, suggesting that kidney damage may have an HIV infection component, probably and endothelial dysfunction.
WYATT et al. (2010) [14] correlated microalbuminuria and mortality risk in HIV patients, even considering advanced HIV diseases. This suggests that maybe microalbuminuria presence could be a non-invasive test for mortality risk.
Another study detected a possible faster progression of microalbuminuria to protein loss in urine in HIV infected patients, and antiretroviral therapy was a remission factor to protein loss. This fact was more impacting in women taking antiretroviral [15] . This is very concerning in our study, since a higher number of women population was not full suppressed with antiretroviral drugs, but we could not find a correlation with higher risk for microalbuminuria or even kidney damage.
5. Conclusions
The present study did not detect a higher level of kidney diseases in a chronic treated HIV population. Besides a microalbuminuria proportion found compared with reviewed articles, it was not correlated with higher risk for kidney damage. We detected a strong correlation of comorbidities presence and age over 50 years with significant kidney function damage. It is important to keep evaluation on this correlation with microalbuminuria getting more patients laboratory results and compare inflammatory markers more specific for endothelial damage.
Kidney diseases are a real serious problem among HIV patients and precocious damage detection and prevention monitoring techniques are recommended to avoid this complication. This is particularly important with high spread use of nephrotoxic drugs. We recommend regular dosage of serum markers and creatinine clearance calculation in clinic follow-up, besides urine analyses. In higher risk individuals, microalbuminuria dosage may be helpful to detect protein loss before kidney damage symptoms.