Equilibria and Stability in Glycine, Tartrate and Tryptophan Complexes, Investigation on Interactions in Cu(II) Binary and Ternary Systems in Aqueous Solution ()
1. Introduction
The naturally occurring form of the acid is L(+)-tartaric acid (H2Ttr) or dextrotartaric acid (figure 1). Tartaric acid is found throughout nature, especially in many fruits and wine [1]. It is added to other foods to give a sour taste, and is used as an antioxidant. Salts of tartaric acid are known as tartrates. As a food additive, Tartaric acid is used as an antioxidant with E number E334. Tartrates are other additives serving as antioxidants or emulsifiers. However, Tartaric acid plays an important role chemically, lowering the pH of fermenting “must” to a level where many undesirable bacteria cannot live, acting as a preservative after fermentation [2]. Tryptophan (figure 1) [1] is one of the 20 standard amino acids, as well as an essential amino acid in the human diet. Tryptophan (Trp) is considered exceptional in its diversity of biological functions [2]. It is a vital constituent of proteins and indispensable in human nutrition for establishing and maintaining a positive nitrogen balance [3]. Besides, some of its derivatives are potent drugs [4]. Trp is widely used in food industry. It is sometimes added to dietary and feed products as a food fortifier in order to maintain the amino acid balance of the food and correct possible dietary deficiencies. Trp can also be used to study structure and dynamics of the proteins because of its indole moiety [5]. In particular, Trp is the precursor of the neurotransmitter serotonin and plays an important role in brain function and related regulatory mechanisms [6]. In
addition, Trp is an important and frequently used starting material in the chemical synthesis of a range of pharmaceuticals [7].
The importance of noncovalent interactions for the shape of macromolecules, the selectivity in biological system is generally accepted and especially hydrophobic and stacking interactions, which have been considered in mixed ligand complexes [8-10].
The distinguishing structural characteristic of tryptophan is that it contains an indole functional group. It is an essential amino acid as demonstrated by its growth effects on rats. Now it is interesting to investigate the complex bilding of ternary systems with Trp. We would like to determine the thermodynamic constants of ternary complexes such as Cu(Har)(L). This kind of structure of L complex can show new aspect of L’s properties in biological systems.
2. Experimental
2.1. Materials
Chemicals were purchased from Merck. Glycine, sodium tartrate, L-tryptophan, copper(II) nitrate trihydrated, sodium nitrate, potassium hydrogen phthalate and standard solutions of sodium hydroxide (titrasol), 2,2’- bipyridyl, 1,10-phenanthroline, nitric acid, EDTA and buffer solutions of pH 4.0, 7.0 and 9.0 were from Merck. All the starting materials were pro analysis and used without further purification. Water was purified by Mili-Q water purification system, deionized and distillated.
2.2. pH Titrations
Reagents: Carbonate-free sodium hydroxide 0.03 M was preparated and standardized against sodium hydrogen phthalate and a standard solution of nitric acid 0.5 mM. Copper (II) nitrate solution (0.03 M) was prepared by dissolving the above substance in water and was standardized with standard solution of EDTA 0.1 M (triplex).
2.3. Apparatus
All pH titrations was performed using a Metrohm 794 basic automatic titrator (Titrino), coupled with a Hero thermostating bath at 25˚C (±0.1˚C) and a Metrohm combined glass electrode (Ag/AgCl). The pH meter was calibrated with Merck standard buffer solutions (4.0, 7.0 and 9.0).
2.4. Procedure
For the determination of acid dissociation constants of the ligand L an aqueous solution (0.3 mM) of the protonated ligand was titrated with 0.03 M NaOH at 25˚C under nitrogen atmosphere and ionic strength of 0.1 M, NaNO3. For the determination of binary (one ligand and Cu2+) and ternary systems (Cu2+, one of the other L ligand (Har) and L), the ratios used were 1:1:1, Cu(II) : L: Har, 0.3 mM. This solution was titrated with 0.03 M NaOH under the same conditions mentioned above. Each titration was repeated seven times in order to check the reproducibility of the data.
2.5. Calculation
The acid dissociation constants,  and
and  for H2(L) were calculated by an algebraic method. The equilibria involved in the formation of 1:1 complex of L and a M (Cu2+, Cu(Bpy)2+, and Cu(Phen)2+) may be expressed as equations (3) & (4).
for H2(L) were calculated by an algebraic method. The equilibria involved in the formation of 1:1 complex of L and a M (Cu2+, Cu(Bpy)2+, and Cu(Phen)2+) may be expressed as equations (3) & (4).
3. Results and Discussion
3.1. Acidity Constants
Ligand (L) can accept one proton on carboxylic group, for which the following deprotonation equilibria hold:
 (1a)
(1a)
 (1b)
(1b)
L can release one other proton from amine group according following deprotonation equilibria:
 (2a)
(2a)
 (2b)
(2b)
Also the two protons in H2(L) are certainly bound at the terminal acetate group and amine group, i.e., it is released from -CO2H or –NH2 according to equilibrium (1) & (2). These values are, as accepted, close to the pKa values of –CO2H which is 2.22 [8].
3.2. Stability of Binary and Ternary Complexes
If we abbreviate for simplicity Cu2+, Cu(Bpy)2+, and Cu(Phen)2+ with M2+, one may write the following two equilibria (3) & (4):
 (3a)
(3a)
 (3b)
(3b)
 (4a)
(4a)
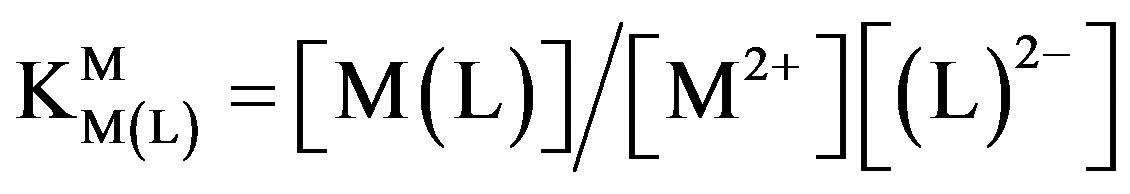 (4b)
(4b)
The experimental data of the potentiometric pH titrations may be completely by considering the above mentioned equilibria (1) through (4), if the evaluation is not carried into the pH range where hyrdoxo complex formation occurs.
The stability of ternary complexes may be evaluated by the following equilibrium:
 (5a)
(5a)
where M is the metal ion, H is the proton, A and B are the ligands. The global stability constants for the ternary complexes may be represented as following:
 (5b)
(5b)
It is possible to define the stability constants for ternary complexes in relation to their binary ones [9], represented by the equilibrium (6) & (7).
 (6a)
(6a)
 (6b)
(6b)
 (7a)
(7a)
 (7b)
(7b)
Differences between the stability constants of the ternary and binary complexes show the tendency of the formation of ternary species [10]. This could be expected by Equation (8):
 (8)
(8)
The difference between the constant refined from experimental data and those calculated statistically using Equation (8) indicates the possibility of ligand-ligand interaction.
3.3. Potentiometric Analyses
The model of species for these ternary systems that was used in superquad program includes all the species of table 1 as well as the hydrolysis of Cu2+ [11,12]. The stability constants of the binary complexes were refined separately using the titration data of this system in a 1:1 and 1:2 ligand: Cu2+ ratio in the same conditions of temperature and ionic strength. They were fixed and, consequently, only ternary species were refined in ternary model of the species. The results are summarized in Table 1. The order of the resulted stability constants are Cu2+ < Cu(Bpy)2+ < Cu(Phen)2+. Figure 2 shows schematic structures of the species with interactions according to equilibrium (4) & (7) for Cu(Phen)(Trp) and Cu(Phen)(Ttr). The results of the acidity constants show good agreement with reported values [13]. The reported stability constant of Cu(L) complex is similar to our results (table 1). The difference between stability constants according equation (8) show that mixed ligand complexes [14-17] formed by a divalent 3d ion, a heteroaromatic N base and an O donor ligand possess increased stability. Now one can calculate the free energy DG, used DlogK received from equation 8 (table 1). We receive for Dlog 5.44 kJ/mol and for Dlog
5.44 kJ/mol and for Dlog 7.34 kJ/mol, which are considerable high. This means that interaction between Cu(Har)2+ and L2- is relative strong and the observed increased stability indicate strong complex bilding of ternary systems.
7.34 kJ/mol, which are considerable high. This means that interaction between Cu(Har)2+ and L2- is relative strong and the observed increased stability indicate strong complex bilding of ternary systems.
It has to be further emphasized that the basicity of the carboxylate group in aqueous solution is very low and consequently this also applies for the coordinating properties of this group.
Comparison of the stability constants for the Cu(Bpy)(L) and Cu(Phen)(L) complexes in table 1 with the corresponding values for Cu(L) indicates an increased stability of the mixed-ligand species. As it is well known for a number of Cu(Har)(L) complexes that an increased complex stability is connected with the formation of intramolecular stack between the aromatic ring systems of 2,2’-Bipyridyl and 1,10-phenanthroline and the heteroaromatic ring of L (opened form « closed

Table 1. Acidity constants of Ttr, Trp, Gly. Logarithm of the stability constants of binary and ternary complexes of M2+ at 25˚C, 0.1 M, NaNO3*.
*The given errors are three times the standard error of the mean value or the sum of the propabable systematic errors, aaccording equation (4), baccording to equation (8).


Figure 2. Schematic structures of the species with interactions according to equilibrium (4) & (7) for Cu(Phen)(Trp) left and Cu(Phen)(Ttr) right. The structure in the right part of the figure was drawn with the program CS Chem 3D, version 3.5, from Cambridge Software Corporation. (Description of colors: C: black, O: red, N: blue, Cu: gray).
form) [10]. The difference between the determined stability constants indicate the experimentally ligand-ligand stack interaction in the Cu(Har)(L) complexes.
As we can see from the experimentally results from Table 1, there is no increased stability constants in case of Cu(Har)(Gly), this means that there is no indication of intramolecular stack interactions. For this reason we can use the stability constants of Cu(Har)(Gly) as opened form in our next calculations.
By employing equation (8) the following definition is possible (equation (9)):
 (9)
(9)
It is evident that the coordination sphere of Cu2+ ions on both sides of this equilibrium are identical, consequently the value for DDlogK is a true reflection of the extent of the intramolecular hydrophobic or stacking interaction in Cu(Har)(L) complexes. The corresponding results are listed in the fourth column of table 2.
Now we can define the intramolecular and thus dimensionless equilibrium constant KI is than given by equation (10) for opened and closed form:
 (10)
(10)
The observed increased complex stability is linked to KI by equation (11):
 (11)
(11)
Knowledge of KI allows calculation of percentage of the macrochelated form according to equation (12) [10]:
 (12)
(12)
The results of the calculations of above mentioned equations are summarized in table 2.
Comparison of the percentage of the macrochelated form according to equation (12) in table 2 shows the high stacking tendency of Trp based on heteroaromatic structure of indole moiety [5].
The distinguishing structural characteristic of tryptophan is that it contains an indole functional group. It is an essential amino acid as demonstrated by its growth effects on rats. Now it is interesting to investigate the complex building of ternary systems with Trp. The comparison of stability constants of these ternary complexes show that Cu(Har)(Gly) exists in open form but Cu(Har)(Trp) is found near 100% in closed form (see last column in table 2). The differences between the stability constants are based on stacked form of Cu(Har)(Trp). The last provides increased stability. The results described in this study show that Trp is a very versatile ligand. Due to the dominating conformation in aqueous solution, hardly any macrochelates are formed in Cu(Har) (Trp) complexes. The energy differences between closed and open form in Cu(Har)(Trp) are significant. One can calculate the free energy DG for Cu(Har)(Trp). So we receive respectively values for Cu(Bpy)(Trp) and Cu(Phen)(Trp) 11.66 kJ/mol and 12.62 kJ/mol. The according structure of ternary Cu(Phen)(Trp) is shown in figure 2. Due to the fact that the resulting data is very interesting, that affects the ternary complexes of Trp in biological systems as active. This might be used, for example in the case of cell separation.
The stability constant of the binary complex was refined separately in the same conditions of temperature and ionic strength. It was in good agreement with reported value [9-14]. Figure 2 shows schematic structures of the species with interactions according to equilibrium (4) & (7) for Cu(Phen)(Trp).

Table 2. Extent of intramolecular stack formation in ternary Cu(Har)(L) complexes as calculated from stability constants (equation (7)). Intramolecular and dimensionless equilibrium constant KI (equation (9)) and percentage of stacked Cu(Har)(L)cl species in aqueous solution at 25˚C, 0.1 M, NaNO3*.
*The given errors are three times the standard error of the mean value or the sum of the propabable systematic errors. afrom table 1, baccording equation (8), caccording equation (9), daccording equation (11), eaccording equation (12).
As we can use from the results in Table 1, the corresponding value for Dlog KCu(Phen)(Ttr) = 0.27 ± 0.13 is relatively high compared with Dlog KCu(Phen)(Gly) = –0.94 ± 0.11. The reason for this increased value of Dlog KCu(Phen)(Ttr) is the involved functional site, such as two carboxyl groups of Ttr in Cu(Phen)(Ttr) complex, compared with one carboxyland one amine groups of Gly in Cu(Phen)(Gly). It is important for the coordination capability of Cu(Phen)(L) complexes, whether the entering ligands Oor N-groups offer. The structure of the ternary complex Cu(Phen)(Ttr) has been shown in figure 2.
NOTES
2Bpy: 2,2’-Bipyridyl.
3Phen: 1,10-phenanthroline.
4Har: Heteroaromatic ligand such as Bpy or Phen.