Sensory denervation with capsaicin reduces ovarian follicular development and delays the onset of puberty in guinea pigs ()
1. INTRODUCTION
An ovary receives its nerve supply from different sources of the autonomic or sensory branches of the peripheral nervous system [1-3]. Some of these sensory neurons are localized in DRGs, specifically from Th11-L5 [2,4,5], and display immunoreactivity for peptides such as calcitonin gene-related peptide (CGRP), substance P (SP), and neuropeptide Y (NPY) [6,7]. Several studies have shown that ovarian afferent nerves, in addition to their basic function in the transmission of sensory modalities from the ovary to the spinal cord, also play an important role in local mechanisms for steroidogenesis, ovulation and vascular tone regulation [8,9].
Furthermore, it has been reported that TRPV1 and NGF receptors (TrkA and p75) are expressed in both sympathetic and sensory fibers that innervate reproductive organs [6,10]. In the ovarian follicles, NGF is synthesized and released from theca and granulosa cells [8] and stimulates follicular development and steroid production [11-13]. In particular, the administration of antibodies to nerve growth factor (NGF Ab) in neonatal rats resulted in failure of the sympathetic nerves due to a reduction in the noradrenaline (NA) level and also produced a partial loss of sensory innervation measured by the CGRP level [8]. In addition, follicular growth was stunted, and the production of progesterone and estradiol was reduced [8]. In immature rats, the same treatment resulted in a delay of the first ovulation and an increase in the plasma LH level [14]. Administration of capsaicin in rats permanently destroyed the sensory fibers, and the proportion of dorsal horn neurons that exhibit slow excitatory transmission was markedly reduced [15], increasing follicular atresia [16] but decreasing estradiol and progesterone secretion from the ovaries [17].
To date, numerous studies on ovarian innervation have shown a close relationship between NA and sympathetic fibers, but a small number of studies have been performed on the sensory fibers and their transmitters in the ovarian system [8,18]. Additionally, these studies used tyrosine hydroxylase (TH), NGF and its receptor TrkA, and p75, and peptides, such as NPY, CGRP, and SP, among others, as markers [13,19,20]. In contrast, the TRPV1 receptors have not been studied in regards to ovarian nerve innervation even though they are essential for sensory nerve transmission. The TRPV1 receptors are localized in both preand paravertebral sympathetic neurons that project to the uterus and ovary [21-23], in the celiac ganglion [24] as well as in sympathetic chain ganglia [25] of neurons supplying the ovaries [1,2,4]. TRPV1 and NGF receptors are functionally interrelated in modulating peptide release and plasticity of peripheral nerve fibers [26- 28]. In uterine cells, the activation of TrkA receptors increases pain sensation mediated by TRPV1 receptors [29], whereas in the urinary bladder cells promote the overactivity response [10]. Additionally, presently there are no reports on the expression of TRPV1 receptors in ovary cells, but these receptors are very important for transmission and cell communication.
The purpose of our study was to investigate the role of ovarian sensory innervation on follicular development and the onset of puberty in the guinea pig. To achieve this goal, sensorial denervation with capsaicin (the specific ligand for TRPV1 receptors) was performed at P10. Here, we tested the hypothesis that ovarian follicular development and the onset of puberty are modulated by sensory fibers mediated by TRPV1 receptors. At first vaginal opening, we evaluated follicular development by light microscopy and TRPV1 receptors by immunocytochemistry in the ovaries, DRGs and dorsal lumbar spinal cord (L2-L4). DRGs and the dorsal lumbar spinal cord (L2-L4) are afferent cellular structures from the ovaries to the SNC [2,4,5]. Partial results were published at a neuroscience meeting in Washington DC, USA [30].
2. MATERIALS AND METHODS
2.1. Animals and Treatment with Capsaicin
All of the procedures described in this work were approved by the BUAP Animal Care Committee and adhered to governmental guidelines (Mexican Council for Animal Care, Norma Oficial Mexicana NOM-062-ZOO- 1999). The local guidelines were also in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals of the USA. All efforts were made to minimize animal suffering and to reduce the number of animals used. The Hartley guinea pigs (Cavia porcellus) were housed in groups (4 animals per cage) under a dark-light cycle of 12 - 12 h with a room temperature of 22 ± 2 degrees Celsius. The animals had free access to food and potable water, which was supplemented with vitamin C.
We used twenty-four female guinea pigs randomly divided into four groups (n = 6). The animals of the first and second group were used for ovary collection, sampling of DRGs and lumbar dorsal spinal cord (L2-L4) for immunocytochemistry and evaluation of the expression of TRPV1 receptors with basal conditions at P10 or FVO. The animals of the third group were administered capsaicin (30 mM) by subcutaneous injection in the upper part of the back at P10, and the animals of the fourth group were administered vehicle (1% ethanol and 99% isotonic saline solution, pH 7.4), and samples were collected at FVO. FVO was defined as the complete loss of the vaginal membrane. In the guinea pig strain used under the described environmental conditions, FVO occurred at 36 days of age (P36) and marked the onset of puberty. At P10 or FVO, the experimental guinea pigs were sacrificed with CO2.
2.2. Immunohistochemistry and Semiquantification of the TRPV1 Receptors in Ovaries, DRGs and Lumbar Dorsal Spinal Cord (L2-L4)
The animals from each experimental group were sacrificed with CO2 and perfused with intracardiac saline solution (NaCl, 0.9%) followed by paraformaldehyde (4%) in PBS, pH 7.4 (PF-PBS solution). The ovaries, DRGs (L2-L4) and lumbar spinal cord (L2-L4) were removed and post-fixed in PF-PBS solution, subsequently embedded in paraffin and then sectioned with a Leica SM2010R microtome (5 µm) for immunohistochemistry. The sections were treated with xylene and ethanol and then washed with PBS solution. The sections were blocked with bovine albumin (IgG-free), treated with Triton X-100 and then washed with PBS at 24 degrees Celsius. The samples were incubated with the anti-capsaicin receptor (1:50, Millipore, USA) overnight at 4 degrees Celsius and then incubated with a secondary antibody (FITC) (IgGfree from goat; 1:250, Millipore, USA) for 2 hours at room temperature. To visualize the nucleus, the sections were incubated with propidium iodide (1:3000, Millipore, USA) for 2 minutes. The specificity of the TRPV1 antibody was confirmed in separate experiments with additional negative controls, including tissue sections incubated in the absence of primary antibody. All sections were mounted on microscope slides with mounting fluid (Millipore, USA) and then observed under a Leica fluorescence microscope.
We analyzed ovary, DRG or spinal cord tissue sections from five different animals with three repeat analyses for each structure. The images were captured with a Leica-DFC325 camera, and the data were stored on the PC hardware. For five different tissues, we counted the positive cells in five representative fields of each tissue section using the cell counter tool from NIH ImageJ software. From a qualitative point of view, the following three levels of fluorescence intensity were identified: light, medium and high. The cells were considered positive when they had a strong green signal, and negative cells were those that had a light or medium green signal. The final quality score was assessed using the software measure tool; the cells were positive when the measure was ≥ 50 arbitrary units, and the cells were negative when the measure was ≤ 49 arbitrary units.
2.3. Morphological Analysis and Amount of Follicles in the Ovary
The ovaries used for immunohistochemistry were also used for morphological examination and to count the follicles. The ovaries embedded in paraffin were serial sectioned with a microtome in 5 µm histological slides; collecting three histological slices until complete ovary section. The histological sections were stained with hematoxylin-eosin and treated by common procedures. All sections were mounted on microscope slides with synthetic resin (Sigma, USA) and subsequently observed under a Zeiss light microscope. The images were captured with a Canon camera, and data were stored on the PC hardware. For morphological examination and ovarian follicle classification, we used previously published criteria [31,32] grouping the ovarian follicles into four categories; healthy preantral follicles (HPF), healthy antral follicles (HAF), atretic preantral follicles (APF) and atretic antral follicles (AAF).
2.4. Statistical Analysis
Data on the number of follicles, the number of TRPV1- positive cells and the onset of puberty age were analyzed using ANOVA followed by the Mann-Whitney U-test. All data were expressed as the means ± SEM, and when the probability was less than 5%, it was considered significant.
3. RESULTS
3.1. Differential Expression of TRPV1 Receptors in the Ovary, DRGs and Lumbar Spinal Cord (L2-L4) of Untouched Guinea Pig at P10 vs. FVO
TRPV1 receptors were localized in the theca-interstitial cells of the ovary follicles, predominantly in healthy antral follicles (Figure 1(a)). TRPV1-positive theca-in terstitial cells in healthy preantral and atretic follicles (preantral and antral) were also detected; however, the immunofluorescent signals were very low. We also found blood vessel positive cells (data not showed). At P10, in healthy antral follicles, the immunofluorescent signal for TRPV1 receptors was low, but was increased 188% above control at FVO (Figure 1(b)) (*p < 0.05, ANOVA followed by a Mann-Whitney U-test). In DRGs, we detected TRPV1-positive small, medium and big neurons (Figure 1(a)) in a proportion of 4:2.1. At P10, the number of TRPV1-positive small, medium and big neurons was low and increased 121%, 100% and 91% at FVO, respectively (Figure 1(c)) (*p < 0.05, ANOVA followed by a MannWhitney U-test). In lumbar spinal cords (L2-L4), the TRPV1 immunofluorescent signal was abundant in dorsal horn region neurons, but was not limited to this area as immunofluorescent cells were detected in the peripheral white matter of the spinal cord where there are more unmyelinated than myelinated axons (Figure 1(a)). As before, the expression of TRPV1 receptors in ovary and DRG cells in the spinal cord of guinea pigs was lower (less positive neurons) at P10 than at FVO (increasing 103%) (Figure 1(d)) (*p < 0.05, ANOVA followed by a Mann-Whitney U-test).
3.2. Capsaicin Delayed the Onset of Puberty
In the guinea pigs injected with capsaicin, the FVO was delayed and occurred at 44 ± 1.8 days of age versus 36 ± 3 days of age in the animals injected with vehicle (Figure 2) (*p < 0.05, ANOVA followed by a MannWhitney U-test).
3.3. Capsaicin Decreased the Ovarian Follicular Development
In comparison to the animals injected with the vehicle, the numbers of HPFs, APFs, HAFs, and AAFs in the ovaries of guinea pigs injected with capsaicin decreased 50%, 66%, 27% and 52%, respectively (Figure 3(a)) (*p < 0.05, ANOVA followed by a Mann-Whitney U-test). A similar result was obtained when the ovarian follicles were grouped only as healthy or atretic follicles. In this case, the number of healthy follicles and atretic follicles
 (a)
(a) (b)
(b)
Figure 1. Immunohistochemistry for the TRPV1 receptors in ovaries, DRG (L2-L4) and lumbar dorsal spinal cord (L2-L4) at P10 and FV0 of untouched guinea pigs (a), Immunohistochemistry for the TRPV1 receptors in tissues of the guinea pig (at P10 and FVO). We show the cell nucleus in red, stained with propidium iodide (AA, AB, AC, AD, AE and AF), TRPV1 receptors in green, marked with FITC (AG, AH, AI, AJ, AK and AL), and the merged images (AM, AN, AO, AP, AQ, and AR). In ovary, theca-interstitial cells (TI) of the healthy antral follicles and granulosa cells (G). DRG (L2-L4): big (white arrow), medium (open arrow) and small neurons (arrowhead). Lumbar dorsal spinal cord neurons (DN). Graphic illustration of the number of TRPV1-positive cells in the ovaries (b), DRG (c) and lumbar dorsal spinal cord (d). The data are presented as the means and SEM (n = 5). Scale bar is equivalent to 10 µm,*p < 0.05, ANOVA followed by Mann-Whitney U-test.
decreased 37% and 57%, respectively (Figure 3(b)) (*p < 0.05, ANOVA followed by a Mann-Whitney U-test).
3.4. Capsaicin Decreased the Expression of TRPV1 Receptors in Ovarian Follicle Cells, DRGs and Lumbar Spinal Cord (L2-L4) Neurons
To determine any changes in expression of the TRPV1 receptor after capsaicin administration, we localized TRPV1 receptors in the ovary cells, DRGs and lumbar dorsal spinal cord neurons of guinea pigs injected with capsaicin or vehicle by immunocytochemistry (Figure 4(a)). In comparison to animals injected with vehicle, the number of TRPV1-positive theca-interstitial cells in the healthy antral follicles decreased 58% in the ovary of guinea pigs injected with capsaicin (Figure 4(b)) (*p < 0.05, ANOVA followed by a Mann-Whitney U-test). A similar result was obtained in small, medium and big DRG neurons where the number of TRPV1-positive neurons was reduced 47%, 17% and 34%, respectively (Figure 4(c)) (*p < 0.05, ANOVA followed by a Mann-Whitney U-test). In the lumbar spinal cord (L2-L4) of the animals injected with capsaicin, the number of TRPV1-positive neurons decreased 44% (Figures 4(a) and (d)) (*p < 0.05, ANOVA followed by a Mann-Whitney U-test).
4. DISCUSSION
In this report, we showed the effects of sensory denervation by capsaicin on ovarian follicular development and the onset of puberty of guinea pigs. Furthermore, we evaluated the expression of TRPV1 receptors in the ovary cells, DRGs and lumbar dorsal spinal cord (L2-L4) neurons by immunocytochemistry in basal conditions (untouched animals) and after capsaicin injection or ve-
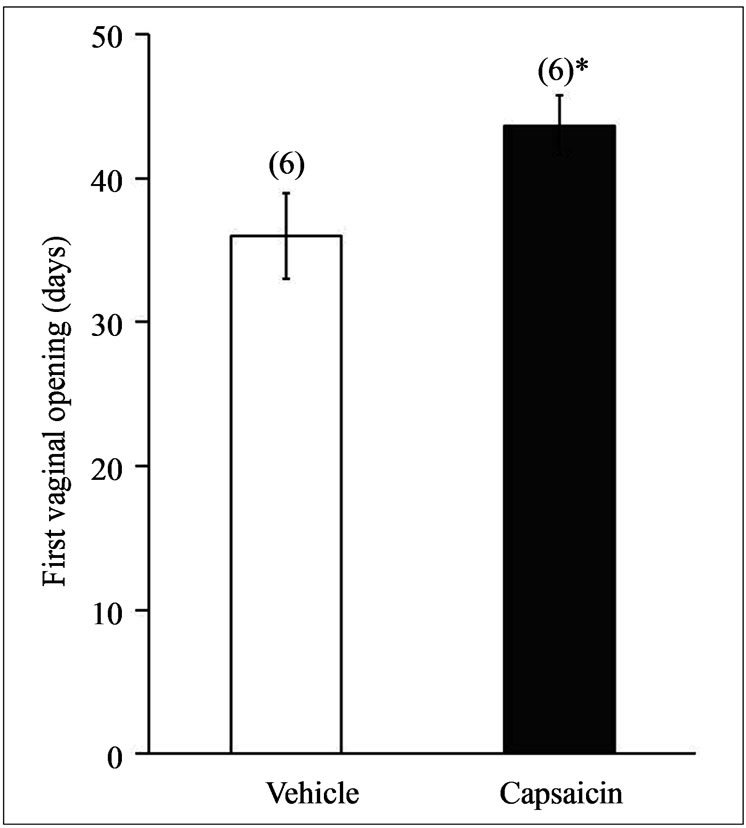
Figure 2. The FVO of the guinea pigs treated with capsaicin. FVO in animals administered vehicle or capsaicin (30 mM) by subcutaneous injection with at P10, *p < 0.05, ANOVA followed by Mann-Whitney U-test.
 (a) (b)
(a) (b)
Figure 3. Number of the follicles in ovary of the guinea pigs treated with capsaicin. Ovarian follicles in animals administered vehicle or capsaicin (30 mM) by subcutaneous injection at P10 and autopsied at FVO. (a) Healthy preantral follicles (HPF), atretic preantral follicles (APF), healthy antral follicles (HAF) and atretic antral follicles (AAF). (b) Healthy and atretic follicles of the ovaries, *p < 0.05, ANOVA followed by MannWhitney U-test.
hicle. These data are the first evidence of the presence of TRPV1 receptors in ovary cells and their participation in follicular development and the onset of puberty.
The fact that the FVO was delayed in the guinea pigs treated with capsaicin suggests that sensory fibers have a role in the hypothalamus-pituitary-ovary axis, which contributes to the onset of puberty in these animals. Previously, it was reported that capsaicin treatment in neonatal rats did not altered the onset of puberty, which was measured by the onset of first estrous; however, the number of ovarian follicles (preantral and antral) and estradiol in the serum of the neonatal rats decreased after neurotoxin administration [16]. The disagreement of our results with this report in rats could be due to the different animal models used because guinea pigs and rats exhibit fundamental differences in peripheral innervation. Guinea pigs have abundant peripheral innervation in visceral organs in contrast to rats, which have scarce peripheral innervation [33,34]. Additionally, guinea pigs have shown high responses to the induction of chemical denervation [35]. In our study, the FVO delay was in concordance with a reduced number of preantral and antral follicles obtained in the guinea pig ovaries treated with capsaicin. Although, estradiol was not measured, we can infer a diminution in its concentration because the antral follicles were reduced, which are the principal source of this steroid hormone [11-13]. This interpretation is in agreement with data obtained in the rat by other authors; denervation induced with capsaicin was found to reduce estradiol in the serum [16]. Additionally, it has been reported that follicular development and ovarian steroidogenesis are regulated by growth factors, such as NGF. This was indicated by a reduction in the transition of preantral to antral follicles in response to NGF Ab treatment [8], a response that was mediated by low affinity receptors (p75NGFR) localized in the theca cells of the follicles [18,36], where NGF also increased FSHRs to stimulate follicular development [12,37]. The reduced number of follicles obtained in the guinea pigs after capsaicin administration, was linked with a reduced number of TRPV1-positive theca-interstitial cells in the healthy antral follicles.
These data support the hypothesis that capsaicin causes a primary failure in follicular development and a subsequent reduction in steroidogenesis. Ovarian thecainterstitial cells are essential to steroidogenesis because theca cells provide androgen substrates for aromatization and estradiol production by granulosa cells. Theca cells express intense staining for 3 beta-hydroxysteroid dehydrogenase (3 beta-HSD), an enzyme required for the biosynthesis of steroid hormones in immature and adult rats during follicular development [38]. Likewise, in thecainterstitial cells, growth factors such as NGF, activate androgen production and stimulate follicular development [11,12]. Also, NFG has been suggested as an activator of androgen production in induced polycystic ovaries in rats [13]. The reduced follicular development and the delayed onset of puberty obtained in the guinea pigs treated with capsaicin could also be the result of a decreased number of TRPV1-positive neurons in the DRGs and in the lumbar dorsal spinal cord, which was indicated by a reduction in the DRG small neurons and the neurons of the substantia gelatinosa (SG; lamina II) of the spinal cord. The DRG small neurons are C primary fibers, the principal group of sensory neurons [39,40]. These results suggest
 (a)
(a) (b)
(b)
Figure 4. Immunohistochemistry for the TRPV1 receptors in ovaries, DRG and lumbar dorsal spinal cord (L2-L4) of guinea pigs treated with capsaicin Immunohistochemistry for the TRPV1 receptors in tissues of the animals administered vehicle or capsaicin (30 mM) by subcutaneous injection at P10 and autopsied at first vaginal opening (FVO). (a) We show the cell nucleus in red, stained with propidium iodide (AA, AB, AC, AD, AE and AF), TRPV1 receptors in green, marked with FITC (AG, AH, AI, AJ, AK and AL), and the merged images (AM, AN, AO, AP, AQ and AR). Ovary theca-interstitial cells (TI) of healthy antral follicles and granulosa cells (G). DRG (L2-L4): big neuron (white arrow), medium (open arrow) and small neurons (arrowhead). Lumbar dorsal spinal cord neurons (DN). Graphic illustration of the number of TRPV1-positive cells in the ovaries (b), DRGs (c) and lumbar spinal cord (d). The data are presented as the means and SEM (n = 5). Scale bar is equivalent to 10 µm,*p < 0.05, ANOVA followed by Mann-Whitney U-test.
that, in guinea pigs, the denervation induced by capsaicin blocks the afferent transmission to supra-spinal structures of the CNS. This interpretation was reinforced by the fact that FVO occurred later when the animals were treated with capsaicin. Furthermore, in the ovaries of the untouched guinea pigs, the number of TRPV1-positive cells was significantly elevated at FVO, suggesting that the ovarian sensory fibers are highly developed at the onset of puberty.
Because the expression of TRPV1 receptors was unknown in the ovary, another focus of our study was to evaluate the expression of TRPV1 receptors in basal conditions by immunocytochemistry. We also evaluated the level of TRPV1 transcripts by RT-PCR amplification (data not yet published). The TRPV1 receptors were found in the theca-interstitial cells of the follicles, with low expression at P10 that increased at the onset of puberty (FVO). These results are consistent with the expression of TRPV1 receptors in many tissues depending on cell type and activity [41,42]. In retina cells and pulmonary arterial smooth muscle cells, TRPV1 receptors promote cell proliferation and migration by MAPK intracellular signal activation [43,44]. In the ovary, this mechanism may be possible, but it has not yet been tested. In DRGs, we also found a high number of TRP-V1-positive small neurons in contrast to medium or big positive neurons, and the TRPV1 expression also increased at FVO. These data are in agreement with previous studies, most likely because small neurons are C-fibers, the major population of sensory nerves [45]. Similarly, the high number of TRPV1-positive neurons that we observed in the dorsal horn of the spinal cord has been previously obtained because the dorsal horn is the anatomical structure where afferent terminals converge on the way to the spinal cord [46]. The expression of TRPV1 receptors was high in DRG small neurons in rats [47] and decreased with capsaicin, specifically in small C and A-delta fibers; that expression that has been restored with growth factors, such as NGF, in previous studies [26]. Previously, it has been suggested that DRG and dorsal spinal cord (T12-L5) neurons are important for the connection between the ovary and the supra-spinal structures of the CNS [3,5].
Additionally, it has been demonstrated that TRPV1 and NGF receptors are co-expressed in sensory fibers and are functionally interrelated through the modulation of the development of neurons, peptide release (CGRP, SP) and sensation of the different qualities that activate primary afferent fibers [28,48]. Although, the effects of denervation on follicular development were evaluated, we can also infer changes in the neurochemical organization pattern of the ovarian sensory fibers due to the lower number of TRPV1-positive neurons in DRG and spinal cord after capsaicin administration. It is recognized that, in sensory fibers, the activity of TRPV1 receptors is augmented by NGF, increasing their plasticity and spontaneous activity [48-50]. Sensory fiber responses decreased with capsaicin and were restored with NGF [42]. In this manner, cell proliferation is decreased in carcinoma tumors when NGF is blocked, a response that is mediated by TRPV1 receptors [50].
Our data suggest that the sensory fibers modulate follicular development and the onset of puberty through TRPV1 receptors, and these receptors can be integrative sensors in the transmission of sensory modalities from the ovary to the CNS.
5. CONCLUSION
For the first time, we showed that, through TRPV1 receptors, sensory fibers modulate ovarian follicular development and the onset of puberty of the guinea pig.
6. ACKNOWLEDGEMENTS
This work was supported by VIEP-BUAP grants 2011-12, and Victorino Alatriste was supported by Consejo Nacional de Ciencia y Tecnología through the doctoral program, CONACYT/45680. We are grateful to Diego Luna for his English language editing.
LIST OF ABBREVIATIONS
CGRP: Calcitonin gene related peptide DRGs: Dorsal root ganglion FVO: First vaginal opening NGF: Nerve growth factor NPY: Neuropeptide Y SP: Substance P TRPV1: Transient receptor potential vanilloid type 1 trkA: Tyrosine kinase A receptors
NOTES
Authors’ contributions: All authors read and approved the final manuscript.