Socio-demographic, host and clinical characteristics of people with typhoidal and non-typhiodal Salmonella gastroenteritis in urban Bangladesh ()
1. INTRODUCTION
Serotypes Salmonella enterica serover Typhi and Salmonella enterica serover Paratyphi A and B are known to cause enteric (typhoid) fever (Typhoidal Salmonella; TS) in human population [1]. The other serovers (non-typhiodal Salmonella or NTS) can colonize the gastrointestinal tract and often causes self-limited acute gastroenteritis [2,3]. The mode of entry is the same for both serovers, i.e. they enter the gastrointestinal system through contaminated food or water. The illness due to TS manifests mostly as gastrointestinal symptoms, such as anorexia (55%), abdominal pain (30% - 40%), nausea (18% - 24%), vomiting (18%) and diarrhea (22% - 28%) [1]. The severity of the illness depends on the virulence of the infecting strain, the inoculum size and host immunity [4]. NTS is generally self-limited; however, may be associated with serious complications such as bacteraemia, meningitis, arthritis or osteomyelitis [5-7]. Among children, it usually causes acute gastroenteritis which due to NTS varies between 3.3% and 4.1% [8-11].
Most of the recent studies observed gastroenteritis in association with infections due to NTS, and have identified young children and immunocompromised individuals at higher risk of infection [11,12]. Case-control studies have compared bacteraemia with non-bacteraemia [7]. Most of the earlier studies analysed blood culture proven typhoidal Salmonella [5,6]. Detection of typhoidal Salmonella in stool specimens is very low and is not routinely performed. Yang MT et al. (1994) reported 146 cases out of 180 (81%) with mean age of 14.8 months suffering from acute gastroenteritis due to TS [13]. Another retrospective analysis in Taipei reported detection of 64 cases of salmonellosis; and of them 66% were pathogenic Salmonella enterica serover Paratyphi B [14]. Conversely, there is a lack of information on Samonella gastroenteritis (both typhoidal and non-typhoidal) from Bangladesh. Taking the advantage of a large Diarrheal Disease Surveillance System (DDSS) database of International Centre for Diarrheal Disease Research, Bangladesh (icddr,b), the present study compared the clinical features, and socio-demographic and host characteristics of gastroenteritis due to TS and NTS as well as their seasonal variations at the Dhaka Hospital of icddr,b in urban Bangladesh.
2. METHODOLOGY
2.1. Study Site, Population, and Source of Data
Established in 1962, the Dhaka Hospital of icddr,b is located in Dhaka, the capital city of Bangladesh. The hospital provides care and treatment to people with diarrheal diseases, who mostly come from urban and periurban Dhaka. During the last 20 years, the hospital has provided cost free care and treatment to over 140,000 patients each year. The DDSS has been established in 1979, which systematically sampled patients—4% of all patients from 1979 through 1995, followed by 2% of all the patients since 1996. The DDSS currently collects information on clinical, epidemiological and demographic characteristics; feeding practices, particularly of infants and young children; and fluid and drug therapy received in homes of every 50th patient, irrespective of age, sex, disease severity or socioeconomic status by administering a structured questionnaire. A trained research assistant interviews the patients, and caregivers in case of young children. Extensive microbiological assessments of freshly collected fecal samples (microscopy, culture and Enzyme-linked immunosorbent assay) are routinely performed to identify diarrheal pathogens. For the present analysis, we extracted relevant information from the electronic database of the DDSS for the period 1993 to 2012.
2.2. Sample Frame
In total, 33,789 children aged 0 - 14 years and 21,075 individuals aged 15 years or older were enrolled in the DDSS from 1993 through 2012. We excluded those with co-infections with other pathogens, such as Shigella, Vibrio cholerae, E. histolytica, G. lamblia, enterotoxigenic E. coli., Aeromonas spp., Campylobacter spp., and rotavirus from our analyses. Finally among children, 54 (0.2%) had TS and 199 (0.6%) had NTS and among the older people 65 (0.3%) had TS and 239 (1.1%) had NTS as confirmed by stool culture. Randomly selected individuals with diarrhea excluding all the pathogens as comparison or control group where n were 253 and 304 for 0 - 14 years and 15 years and above respectively (Figure 1).
2.3. Definition
Diarrhea was defined as passage of three or greater number of abnormally loose or watery stools in the preceding 24 hours [15]. Individuals with no formal schooling were considered as illiterate. We defined pneumonia as presence of adventitious sound (rhonchi or crepitation) in the lung [16]. Fever was defined as axillary temperature more than 37.8˚C. Malnutrition was defined following WHO guidelines wasting (weight-for-height z-score < −2.00 SD), stunting (height-for-age z-score < −2.00 SD), and underweight (weight-for-age z-score < −2.00 SD)] [17].
2.4. Meteorological Data
Data on daily maximum and minimum temperatures, rainfalls, relative humidity and sea level pressures were obtained from Dhaka station of Bangladesh Meteorological Department. Summary of monthly and yearly meteorological data were calculated from the daily records.
2.5. Specimen Collection and Laboratory Procedures
A single, fresh stool specimen (at least 3.0 mL or grams) was collected from each of the patients and immediately sent to icddr,b’s central Clinical Microbiology Laboratory for detection of TS and NTS following standard methodology [18].
2.6. Data Analysis
Data analyses were done by using Statistical Package for Social Sciences (SPSS) Windows (Version 15.2; Chicago, IL) and Epi Info (Version 6.0, USD, Stone Mountain, GA). For categorical variables, differences in the proportions were compared by Chi-square test and a probability of <0.05 was considered as statistically significant. Strength of association was determined by estimating odds ratio (OR) and its 95% confidence interval (CI). Associations between culture positive TS and NTS in stool specimen (outcome) and each of the variables of interest were assessed by way of odds ratios (OR), using 2/2 table as well as logistic regression analysis. Finally, cumulative value of TS and NTS was compared with comparison group or individual with no pathogen detected in stool culture both in univariate as well as multivariate analysis.
2.7. Ethical Consideration
The DDSS was approved by the RRC (Research Review Committee) and ERC (Ethical Review Committee) of icddr,b. Patients were enrolled and their stool specimens collected for various laboratory tests only after they/their parents (in case of minors) provided verbal informed consent.
3. RESUTLS
Among the culture positive TS cases (n = 119), 78 (65%) were Salmonella enterica serover Typhi, 21 (18%) were Salmonella enterica serover Paratyphi B and 20 (17%) were Salmonella enterica serover Paratyphi A. Out of these total 119 patients, 54 (45%) were 0 - 14 years of age and 65 (55%) were 15 years of age or older. There were a total of 438 patients infected with NTS after excluding those with proven co-pathogens. Of these 438 patients, 199 (45%) were in the 0 - 14 years age group, and the remaining 239 (55%) were 15 years of age or older (Figure 1).
With the exception of presence of fever in greater proportion of TS patients, the clinical features between TS and NTS groups were similar. The proportion of patients with underweight, wasting and stunting was higher among TS patients but the differences with NTS group were not statistically significant (Table 1). Stool microscopic examination revealed identical distribution of red blood cell (RBC), and inflammatory cells (fecal leukocyte and macrophage) in both the groups. However, TS individuals more often had alkaline stool pH compared to individuals with NTS infection (Table 1). Meanwhile, lower proportion of individuals with TS use and drink unboiled water (Table 1).
Significant differences were observed with the cumulative observation of TS and NTS with control group among 0 - 14 years. TS and NTS belonged to lower socio-economic families with poor water and sanitation practice compared to individuals without any pathogens. Significant higher proportion of TS and NTS children had fever and pneumonia; however, lower proportion of them presented with watery stool with some or severe dehydration. They were more wasted, underweight and more frequently presented with RBC and inflammatory cells with alkaline stool (Table 1). In multivariate analysis, significant associations were observed with TS and NTS children with lower socio-economic status, unboiled drinking water, fever, pneumonia, wasting, presence of RBC and inflammatory cells in stool and alkaline pH (Table 1).
Among individuals aged 15 years and above, TS were more frequently isolated from poor socio-economic background and user of non-tap drinking water less often than their NTS counterparts. Other socio-demographic indicators and clinical characteristics were found equally distributed in both bi-variate and multivariate analyses (Table 2). On the other hand, comparing the cumulative proportion of TS and NTS, it was revealed that proportion of self lack of formal schooling was lower compared to control or no pathogen detected group. Higher proportion of them had fever, with longer duration of stay in the hospital and often presented with RBC and inflammatory cells in stool; however, higher proportion of individuals without any pathogen in stool needed intravenous saline for initial rehydration (Table 2). In multivariate analysis, self education, fever, duration of stay in the hospital (>24 hours), use of intravenous saline for rehydration and presence of RBC were significantly associated with TS and NTS individuals (Table 2).
Two seasonal peaks of TS were observed during the months of April to June and September to November; however, only one peak was observed in June-August for NTS (Figure 2).
4. DISCUSSION
Compared to NTS, higher proportion of TS patients had fever, and was associated with TS after adjusting for potential confounders. This was an expected observation; TS is typically associated with high fever [1]. Hyperplasia and necrosis of lymphoid follicles involving both mucosa and submucosa, ulceration in the epithelium and often deeper ulcerations sometimes lead to perforation of intestine and/or hemorrhage, and involvement of reticuloendothelial cell and cytokines mediators might cause high rise of temperature in TS [1]. However, such phenomenon is not much clear for NTS which is often responsible for foodborne outbreaks.
There were no statistical differences observed in many of the socio-economic-demographic indicators, such as
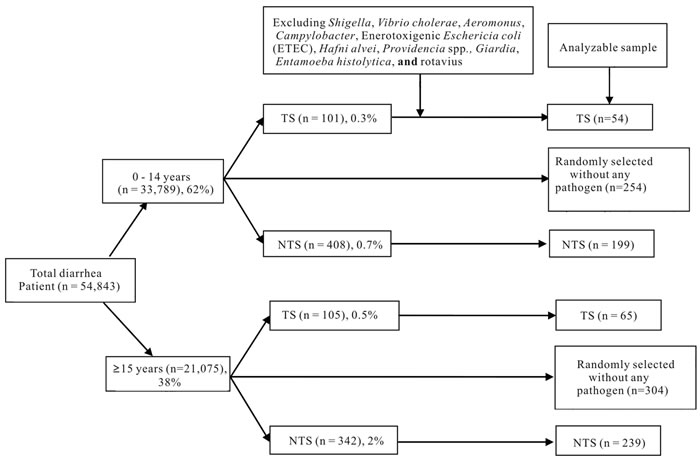
Figure 1. Sample framing (1993-2012). TS: Typhiodal Salmonella; NTS: Non-typhi Salmonella.
maternal and paternal literacy, poor socioeconomic status, use of sanitary toilet and slum dwellings as well as clinical features were different between TS and NTS patients. This could be due to the inadequate sample size in TS group. The impact of maternal literacy an indicator for childhood hygiene practices is well known, which was also observed in the present study [19]. Poor socioeconomic status of people living in the slum area is also associated with poor hygiene practices, use of unsafe water and consumption of contaminated food [20].
Contaminated food and water are the most important modes of TS transmission [21]. In Bangladesh, water and sanitation facilities are not optimal [22] and consumption of relatively cheap unhygienic foods are potentially contaminated by enteric pathogens, like those from the street venders are common. People with poor socio-economic status and living in slums are often exposed to such risks. In the present study, risks for TS infection were lower among those who use non-tap water for drinking, which means chances of contamination of supply water at any point of supply chain. On the other hand, at least 61% of the study population did not drink water which was not properly treated such as boiling. The proportion was really higher compared to control group with diarrhea without any detectable common etiologic agents. Malnutrition associated with immuno-compromised status [23] is more common among city slum dwellers that put them at risk for TS or NTS infections compared to diarrhea sick individuals without any pathogen. With regard to this, higher proportion of lower respiratory tract infection among TS and NTS was observed which might co-exist with malnutrition.
After ingestion, pathogenic Salmonella passes through the gastric acid barrier and invades the mucosa of the small and large intestine. Invasion of epithelial cells stimulates the release of pro-inflammatory cytokines that induces an inflammatory response. The acute inflammatory response causes diarrhea and may lead to ulceration and destruction of the intestinal mucosa [21]. This patho-physiologic process leads to presence of red blood cell, and inflammatory cells (fecal leukocyte, and macrophage) in the stool. In our analyses, we noted higher proportion of patients with TS to have an alkaline stool compared to NTS; however, this became insignificant in the logistic regression. However, significant difference was observed with control group. Human feces are normally alkaline [24], and an acidic stool can indicate a digestive problem such as lactose intolerance; rotavirus infections and some of the diarrhoeagenic E. coli infections may be associated with an acidic stool.
Although at least 85% of patients less than 15 years, presented with watery stool without mucous or blood, it was higher among control group. This finding correlated with higher proportion of control patients who have some or severe dehydration. It is really difficult to explain due to exclusion of all the pathogens commonly responsible for dehydrating diarrhea such as Vibrio cholerae, rotavirus or ETEC; imbalance between fluid loss
 (a)
(a) (b)
(b) (c)
(c)
Figure 2. Monthly distribution of overall typhoidal Salmonella (TS); and non-typhi Salmonella (NTS) (1993-2012).
and intake might not be well maintained by the caregiver or the patient himself.
Seasonal patterns were noted for both the pathogens and their relation with environmental factors such as ambient temperature, rainfall, humidity, and sea level pressures. We noted two distinct seasonal peaks for TS, and a single peak for NTS when TS remains less prevalent, which has also been noted in previous studies [25]. Seasonal upsurge of one pathogen challenging the other might be due to environmental interaction.
5. LIMITATION
The present study was conducted among people attending a hospital, and this population may not be representative of the general population. Moreover, we did not compare the findings of stool culture positive TS cases with those of blood culture positive TS individuals due to absence of information in the database. However, easy access to hospital by people, irrespective of their socioeconomic or other status, systematic collection of information, large sample base, and unbiased sampling of the surveillance system and high quality of performance of our Clinical Laboratory, providing reliable results are other strengths.
6. CONCLUSION
Gastroenteritis caused by TS differs from NTS in terms of febrile response and socio-economic background in under-fifteen and above age group respectively, although other features are comparable in both the groups. Findings of the present study also reveal significant difference between TS and NTS with individuals with diarrhea not infected with any common pathogens. This will help the clinician for case differentiation and rational use of intravenous fluid as well as antimicrobials. Results of the present study will strengthen the knowledge in the field of epidemiology of Salmonella to generate further trials which may help the policy makers in planning interventions for the at risk population especially in the field of water-sanitation and vaccine development and implementation.
7. ACKNOWLEDGEMENTS
Diarrheal Disease Surveillance System (DDSS) of icddr,b was supported for some of the study years by the Government of the People’s Republic of Bangladesh under its IHP-HNPRP. icddr,b acknowledges with gratitude the commitment of the Government of the People’s Republic of Bangladesh to the icddr,b’s research efforts. icddr,b also gratefully acknowledges the following donors which provide unrestricted support to its research efforts: Australian Agency for International Development (AusAID), Government of the People’s Republic of Bangladesh, Canadian International Development Agency (CIDA), Embassy of the Kingdom of the Netherlands (EKN), Swedish International Development Cooperation Agency (Sida), Swiss Agency for Development and Cooperation (SDC), and Department for International Development, UK (DFID). None of the authors have any competing interests. The donors had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
NOTES
#Corresponding author.