Comparison of Two Renal Cell Lines (NRK-52E and LLC-PK1) as Late Stage Apoptosis Models ()
1. Introduction
Continuous renal cell lines are frequently used cell models in toxicology due to their technical and economic advantages over primary cells or in vivo solutions. Especially NRK-52E (rat), LLC-PK1 (pig), MDCK (dog), VERO (monkey), BHK (hamster) and OK (opossum) are used extensively for investigation of mechanisms and signal transduction pathways in toxicology.
The aim of the project presented here was to investigate the suitability of two of these renal cell lines, namely NRK-52E and LLC-PK1, with respect to their sensitivity to various known apoptosis inducers using mid to late stage apoptosis, i.e., chromatin condensation and DNA fragmentation, as endpoints.
Nuclear chromatin condensation and chromosomal DNA fragmentation are well-described key features in apoptosis and are strikingly similar in different cell types [1]. The chromatin condensation is microscopically visible as dense chromatin aggregates typically near to the nuclear membrane, e.g. after staining with certain dyes such as the blue-fluorescent Hoechst 33,342 dye staining condensed chromatin of apoptotic cells more brightly than the chromatin of normal cells. Condensed chromatin with a distinct fragment size, roughly 180 - 200 base pairs and multiples thereof, is the result of a specific DNA fragmentation via cleavage by endogenous endonucleases. The latter DNA fragments can be used as a marker for apoptosis, e.g. via the DNA laddering assay [1]. In contrast, necrosis is typically characterized by random DNA fragmentation resulting in a DNA smear rather than a distinct ladder in DNA agarose gel electrophoresis.
The set of test substances, reportedly used as positive controls for apoptosis induction in in vitro cell systems, included cadmium chloride (CdCl2), dithiothreitol (DTT), sodium chloride (NaCl), mercuric chloride (HgCl2), tributyltin oxide (TBT-O), tributyltin chloride (TBT-Cl) and staurosporine [2-14].
2. Materials and Methods
2.1. Materials
Unless stated otherwise, materials and chemicals were purchased as follows: PAA Laboratories GmbH, Cölbe, Germany (cell culture chemicals), Greiner Bio-One GmbH, Frickenhausen, Germany (cell culture plastics), and Sigma-Aldrich GmbH, Seelze, Germany (all other chemicals).
2.2. Test Substances
Cadmium chloride (CdCl2), dithiothreitol (DTT), sodium chloride (NaCl) and mercury chloride (HgCl2) stock solutions were prepared in deionized water. Tributyltin oxide (TBT-O) and tributyltin chloride (TBT-Cl) were dissolved in absolute ethanol and staurosporine stock solution was prepared in dimethylsulfoxide (DMSO) (Merck, Darmstadt, Germany). All stock solutions were sterilized by filtration (0.2 µm), diluted in the corresponding solvent and added to cell culture medium using a dilution factor of 40. Final solvent concentrations were 0.5% (v/v) for all solvents employed. The latter solvent concentrations were previously shown to have no significant adverse effect on the cell types used (data not shown).
2.3. Cell Culture and Treatments
LLC-PK1 and NRK-52E cells were obtained from the European Collection of Cell Cultures, Salisbury, UK (ECACC # 86121112) and from the DSMZ, Braunschweig, Germany (DSMZ #ACC 199), respectively. Both cell lines were cultured in DMEM supplemented with 10% FBS and antibiotics (with a final concentration of 100,000 U/L penicillin and 100 mg/L streptomycin) under standard conditions (37˚C, 5% CO2) and subcultured twice per week with a dilution ratio of 1:10. Passages 2 - 18 were used for experiments. Cells were seeded at a density of 1 × 104 cells cm−2 in 21 cm2 tissue culture Petri dishes for Hoechst staining and in 175 cm2 flasks for DNA laddering. Incubations (2 to 48 hours) with the test compounds (Table 1) commenced 24 hours after seeding.
2.4. Hoechst Staining
Following compound exposure cell cultures were washed with modified PBS (136.9 mM NaCl, 2.7 mM KCl, 8.1 mM Na2HPO4, 1.5 mM KH2PO4, pH 7,4, 300 mosM) and then fixed with ice-cold absolute methanol for 10 minutes. After 7 minutes incubation with Hoechst 33,342 dye solution (1 µM in PBS) at room temperature, samples were washed again with PBS and a cover-slip was mounted with anti-fading mounting medium (cat# S3023, Dako, Hamburg, Germany). After storage at 4˚C over-night, chromatin condensation was evaluated visually using a microscope equipped with a broad range UV light excitation filter and then photographed for documentation.
2.5. DNA Extraction and DNA-Laddering
The DNA laddering assays were performed according to a previously published method [15] with slight modifications. Briefly, after compound exposure cellular DNA was isolated via phenolic extraction. The purity and amount of DNA isolated was determined spectrophotometrically. DNA samples were separated on a 1.8% agarose gel and visualized after ethidium bromide staining on a UV screen and photographs were taken.
3. Results
3.1. Hoechst Staining
NRK-52E and LLC-PK1 cells were exposed to seven different compounds (Table 1). The concentrations and exposure durations were chosen based on literature data [2-14]. All exposures were performed and analyzed using standard conditions, i.e., in medium supplemented with 10% FBS. Representative microscopic pictures are shown in Figure 1. Interestingly, in LLC-PK1 cells four out of seven compounds, and in NRK-52E all compounds failed to elicit measurable apoptosis (Table 1). In order to elucidate whether the FBS content could be re-

Table 1. Hoechst staining results in LLC-PK1 and NRK-52E cells.

Figure 1. Representative pictures from Hoechst staining experiments with LLC-PK1. (A), untreated; (B), 1 µM staurosporine (12 h); (C), 5 µM TBT-Cl (4 h); bar = 20 µm.
sponsible for these results, the assays were repeated for the non-inducers without FBS addition. In LLC-PK1 cells, two compounds, i.e., cadmium chloride (CdCl2) and mercuric chloride (HgCl2), showed distinct signs of apoptosis, although at rather high concentrations and long incubation times (Table 1). The other two known apoptosis inducing compounds remained negative in the LLC-PK1 cells. In contrast, all test compounds were negative in NRK-52E cells, irrespective of the presence/ absence of FBS in the medium used.
TBT-Cl and TBT-O exposure of the LLC-PK1 cells resulted in ambiguous results with Hoechst staining (Figure 1(C)). Therefore, additional testing was performed using DNA laddering.
3.2. DNA Laddering
TBT-O and TBT-Cl induced clear DNA laddering at low concentrations and short-term exposures in LLC-PK1 cells (Table 2, Figure 2). Under the same conditions, NRK-52E cells showed no laddering with TBT-Cl and only a smear without distinct laddering with TBT-O (Table 2). During exposure of both cell types it could be observed that in a certain number of cells the contact to the surrounding cells and to the substrate was less tight and some cells started floating. During cell harvesting these types of cells are typically lost during washing. In order to test whether an important cell fraction might be lost this way, these cells were specifically collected and analyzed. And indeed, these non-adherent cells from both cell types showed distinct DNA laddering patterns at a minimum of 0.5 µM TBT-Cl and after 2 hours of exposure (Table 2, Figures 2-3).
4. Discussion
This study aimed to investigate the suitability of the renal cell lines NRK-52E and LLC-PK1 for mid to late stage apoptosis research using chromatin condensation and DNA fragmentation as endpoints under standard cell culture conditions, i.e., in the presence of serum. Despite that many other investigators used serum-depleted cells, this was omitted here for two reasons: 1) the presented work should set the basis for future testing using compounds that require binding to serum components for membrane


Figure 2. DNA laddering in non-adherent and adherent LLC-PK1E cells after exposure to TBT-Cl. Left, floating cells; right, adherent cells.

Figure 3. DNA laddering in non-adherent NRK-52E cells after exposure to TBT-Cl.
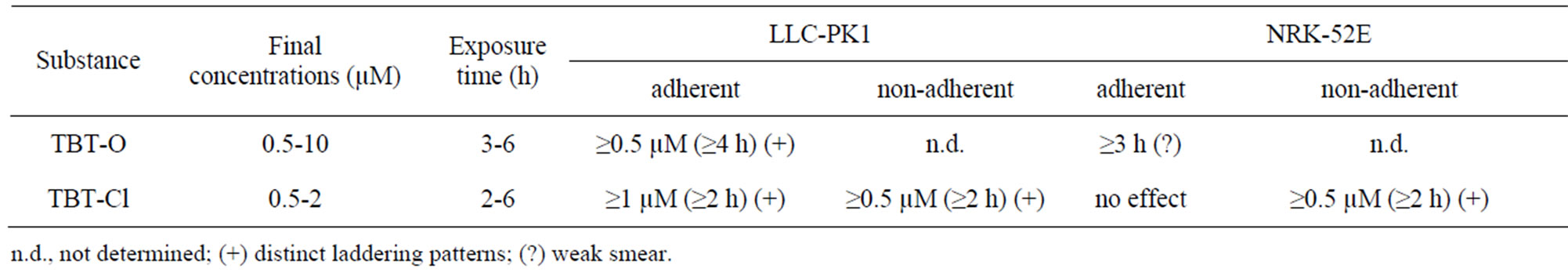
Table 2. DNA laddering results in LLC-PK1 and NRK-52E cells.
transport, i.e., similar to the in vivo situation; and 2) serum deprivation was demonstrated to trigger apoptosis in renal cells [16].
As apoptotic cell death is an extremely complex process, more than one endpoint is necessary to achieve reproducible results. Depending on cell type and apoptotic pathway, not all known features may be observable within one experiment. Also, differentiation between necrosis and apoptosis may be difficult. Correspondingly chromatin condensation and DNA fragmentation were chosen as endpoints in the study presented here, and only substances positively tested in the Hoechst staining experiments were subjected to further testing.
Seven known apoptosis inducers were used in the Hoechst staining assay, i.e., CdCl2, DTT, NaCl, HgCl2, TBT-O, TBT-Cl and staurosporine, but only the last three elicited apoptosis in LLC-PK1 cells when tested under standard conditions (in the presence of serum). TBT-O and TBT-Cl had been previously shown to induce apoptosis using the same methods and at similar concentrations for example in rat thymocyte cultures and Jurkat cells, respectively [7-9]. Staurosporine induced apoptosis after exposure to 100 nM for 4 hours. This is in good agreement with data from other investigators using human glioma cells (U251MG) or human kidney cells (HK-2) [12,13]. The four compounds that elicited no apoptotic response in the Hoechst assay in the presence of serum were retested under serum-deprived conditions whereby CdCl2 and HgCl2 elicited an apoptotic response, albeit only at high concentrations and long incubation times. CdCl2 and HgCl2 induced DNA laddering in LLCPK1 cells was observed previously under similar conditions [5,3], although very high HgCl2 concentrations (≥ 100 µM) were reported to induce necrotic cell death [3].
Hypertonic NaCl was shown to induce caspase-3 mediated apoptosis at ≥550 mosmol kg-1 H2O [2,6,14] in the mouse renal medullary mIMDCD3 cell line. In contrast to the latter, hypertonic NaCl had not apoptotic effect in LLC-PK1 in the presence or absence of serum, in the study presented here.
DTT was successfully used by van de Water et al. in rat renal proximal tubular cells (PTC) resulting in significant DNA fragmentation following exposure to 10 mM for 6 hours [11]. In other cell lines e.g. human leukemia HL-60 cells, late stage apoptosis (DNA fragmentation) was observed already after 3 - 4 hours exposure to 2 mM DTT [10].
Staurosporine induced the typical features of apoptosis in the Hoechst stain, i.e., half-moon-shaped chromatin condensation at the nuclear borders. In contrast, TBT-O and TBT-Cl resulted in ambiguous staining patterns (e.g. disrupted cells and nuclei), which were not readily distinguishable from necrosis. Consequently, TBT-O and TBT-Cl were further tested using the DNA-laddering assay that allowed confirmation of apoptosis.
Contrary to expectations, NRK-52E cells did not respond to known apoptosis inducing compounds, irespective of the concentrations and exposure durations used or the presence/absence of FBS in the media. Despite that this cell line has been frequently reported to be a useful model for understanding the mechanisms underlying apoptosis, albeit using other compounds [17-20], the results obtained in this study presented here shed some doubt as to the suitability of the model to study apoptosis.
When adherent and non-adherent NRK-52E cells were investigated separately using the DNA laddering assay, apoptosis could be detected with TBT-O and TBT-Cl in detached cells only. This suggested that the apoptotic cell fraction may be lost for Hoechst analysis at an early time point during compound incubation due to cell detachment. Although a similar observation was made for LLCPK1 cells, the degree and number of detached cells was not as pronounced as in NRK-52E.
The latter raises the question whether cells that enter apoptosis detach from the surface as a consequence of apoptosis or whether the cells detach from the surface first which then triggers apoptosis. Cells can become detached from their substrate as a result of damage to actin filaments and subsequently enter apoptosis through loss of cell-matrix contacts. This is a well-known feature of renal cells [11] and is also sometimes observed in vivo, for example in ochratoxin-exposed rats [21].
In literature, statements are controversial with respect to the potential of the investigated cell lines to undergo apoptosis, be this a consequence of different strains provided by the cell line collections (ATCC, ECACC, DSMZ etc.) or varying cell culture and exposure conditions of different investigators.
In summary, LLC-PK1 cells, but not NRK-52E cells, are suitable models of mid to late stage apoptosis under the tested conditions. Of the compounds tested, only TBT-O, TBT-Cl and staurosporine were shown to be suitable positive controls for future testing. It can also be concluded from this study that “positive controls” may in fact not work as positive controls in some cell lines and that a thorough establishment of true “positive controls” including their underlying cellular mechanisms is required prior to embarking on the investigation of in induction of apoptosis by some unknown compounds.
5. Acknowledgements
The authors like to thank K. Kobras for her assistance with assay performance.
NOTES