1. Introduction
In recent years, the search for environmentally benign chemical processes or methodologies has received much attention [1-4]. Heterogenization of homogeneous catalysts has been an interesting area of research from an industrial point of view; this combines the advantages of homogeneous catalysts (high activity, selectivity, etc.) with the engineering advantages of heterogeneous catalysts (easy catalyst separation, long catalytic life, easy catalyst regeneration, thermal stability, and recyclability) [5-9]. Application of solid acids in organic transformations is important, because solid acids have many advantages such as simplicity in handling, decreased reactor and plant corrosion problems, and more environmentally safe disposal [5-13]. Protection of functional groups plays an important role in the synthesis of complex organic molecules such as natural products. The protection of alcohols, phenols and amines is an important and frequently utilized transformation in synthetic organic chemistry [14]. Among the various protecting groups used for the hydroxyl and amine groups, acetyl is one of the most common groups due to its facile introduction and its stability in the acid reaction conditions and also easy deprotection using mild alkaline hydrolysis [15]. The most commonly used reagent combinations for this reaction are acetic anhydride or acetyl chloride in the presence of Et3N and pyridine as catalysts [16-18]. 4-(Dimethylamino) pyridine (DMAP) is known to cause remarkable rate acceleration in the reaction [19]. A variety of catalysts such as silica gel supported sulphuric acid [20,21], Ammonium acetate in acetic acid [22], Zinc dust [23], ZnCl2 [24], CoCl2 [25], Sc(OTf)3 [26], TaCl5 [27], Montmorillonit K10 [28], HY Zeolite [29], In(OTf)3 [30], Cu(OTf)2 [31], Yittria-Zirconia based Lewis acid [32], InCl3/Mont. K 10 [33], Manganese (III) bis(2-hydroxyanil) acetylacetonato complex [34], Silica sulfate [35], p-MeC6H4SO2NBr2 [36], DBDMH or TCCA [37], H6P2W18O62·24H2O [38], La(NO3)3·6H2O [39], Ionic liquid based on morpholin [40], Borated zirconia [41], 2,4,6-Triacyloxy-l,3,5-triazine (TAT) [42], Sodium dodecyl sulfate (SDS) [43] and DMAP. saccharin [44] are also known to catalyze the acetylation of alcohols, phenols and amines. However, most of these methods suffer from at least one of the following disadvantages like vigorous reaction conditions, high cost and toxicity of the reagent, tedious work-up procedures, unsatisfactory yields, and instability and hygroscopic nature of the reagent.
Carbon-based solid acid catalysts [45-48] have gained importance due to their significant advantages over homogeneous liquid phase mineral acids, such as increased activity and selectivity, longer catalyst life, negligible equipment corrosion, ease of product separation, and reusability. We, recently developed a simple and fast method for the preparation of a similar sulphonic acid functionalized polycyclic aromatic carbon catalyst from bioglycerol (biodiesel by-product) and also from glycerol-pitch (waste from fat splitting industry) by in situ partial carbonization and sulfonation [49,50]. Such catalysts have been shown to be inexpensive, highly stable, robust, recyclable, and easily produced from naturally available bioglycerol, and are demonstrated to be effective for the esterification of fatty acids to its methyl esters [49], THP protection and deprotection of alcohols and phenols [50], and also for the synthesis of highly substituted imidazoles [51], 3,4-dihydropyrimidin-2-(1H)- ones [52], amides from aldehydes [53], and spirooxindole derivatives [54]. In continuation of our efforts towards exploring the applications of the glycerol-based carbon catalyst, here we report a simple and an efficient methodology for the acetylation of alcohols, phenols and amines with acetic anhydride at 65°C under solvent-free conditions in excellent yields (Scheme 1).
2. Experimental Details
All chemicals and reagents were procured from suppliers and used without further purification. The isolated products were characterized by chromatographic and spectral studies (GC, GC-MS, FT-IR and 1H NMR). The spectra were compared with those of standard esters. The NMR spectrums of product were obtained using Bruker AC- 300 MHz spectrometer with TMS as the internal standard.
2.1. General Experimental Procedure for the Preparation of Glycerol-Based Carbon-SO3H Catalyst
Carbon-SO3H catalyst was prepared as reported [49] by heating a mixture of glycerol (10 g) and concentrated sulfuric acid (30 g) from ambient temperature to 210°C - 220°C for 20 min, to facilitate in situ partial carbonization and sulfonation. The reaction mixture was allowed to remain at that temperature for about 5 min (until foaming ceased) to obtain the carbon material. The solid material was cooled to ambient temperature and washed with hot water under agitation until the wash water showed a neutral pH value. The partially crystalline product was filtered and dried in an oven at 120ºC for 2 h until it was moisture free to obtain the glycerol-based carbon acid catalyst (4.67 g).
2.2. General Experimental Procedure for the Catalytic Acetylation of Alcohols, Phenols and Amines
To a stirred mixture of the alcohol/amine (1 mmol) and Ac2O (2 mmol), carbon catalyst (15 wt% of substrate) was added and stirring continued at 65°C for 30 to 120
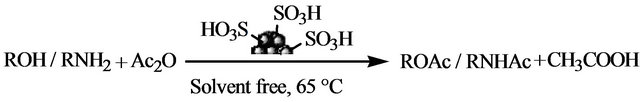
Scheme 1. Acetylation of alcohols, phenols and amines employing carbon-SO3H catalyst.
min. The progress of the reaction was monitored by TLC. After completion of the reaction, ethyl acetate (3 × 5 mL) was added to the reaction mixture and the catalyst was separated by filtration. The organic phase was washed with saturated NaHCO3 solution (15 mL), dried over anhydrous Na2SO4 and concentrated under reduced pressure in a rotary evaporator to afford the crude product. The final product was purified by silica gel column chromatography using hexane/ethyl acetate as eluting solvents. The yield was calculated as mmol of purified product with respect to mmol of initial alcohol/amine. The reaction times and yields of the products are presented in Tables 1 and 2. All the purified products were characterized by GC, IR and 1H NMR studies and the data is in comparison with authentic samples.
1-Octyl acetate (Entry 1, Table 1), 1H NMR (300 MHz, CDCl3) δ 0.88 (t, 3H), 1.20-1.43 (m, 10H), 1-65-1.78 (m, 2H), 2.04 (s, 3H), 4.05 (t, 2H). IR (cm−1): 2928, 2858, 1742, 1238 1039. GC-MS m/z: 172 [M]+.
1-Decyl acetate (Entry 2, Table 1), 1H NMR (300 MHz, CDCl3) δ 0.88 (t, 3H), 1.20 -1.45 (m, 30H), 2.0 (m, 2H), 2.04 (s, 3H), 4.05 (t, 2H). IR (cm−1): 2925, 2854, 1741, 1239. GC-MS m/z: 200[M]+.
1-Octadecyl acetate (Entry 3, Table 1), 1H NMR (300 MHz, CDCl3) δ 0.89 (t, 3H), 1.20 -1.45 (m, 30H), 2.0 (m, 2H), 2.04 (s, 3H), 4.05 (t, 2H). IR (cm−1): 2928, 2858, 1742, 1238, 1039. GC-MS m/z: 312 [M]+.
2-Ethyl-1-hexyl acetate (Entry 4, Table 1), 1H NMR (300 MHz, CDCl3) δ 0.9 (t, 6H), 1.2-1.4 (m, 7H), 2.1 (s, 3H); 3.9 (t, 2H). IR (cm−1): 3020, 2962, 1726, 1215, 763. GC-MS m/z 172 [M]+.
Cyclohexyl acetate (Entry 5, Table 1), 1H NMR (300 MHz, CDCl3) δ 1.25-1.85 (m, 10H), 2.00 (s, 3H), 4.65 (m, 2H). IR (cm−1): 3020, 2940, 1721, 1256, 1215, 756. GC-MS m/z: 142 [M]+.
2-Ethoxy ethyl acetate (Entry 6, Table 1), 1H NMR (300 MHz, CDCl3) δ 1.23 (t, 3H), 2.04 (s, 3H), 3.40 (m, 2H), 3.58 (t, 2H), 4.51 (t, 2H). IR (cm−1): 3020, 2962, 1726, 1215, 763. GC-MS m/z: 132 [M]+.
Acetylated sun flower Sterols (Entry 7, Table 1), 1H NMR (300 MHz, CDCl3): δ 0.70 (s, 3H), 0.88 (d, 6H), 0.94 (d, 3H), 1.04 (s, 3H), 2.05 (s, 3H), 4.64 (m, 1H), 5.39 (d, 1H). IR (cm−1): 2940, 1730, 1262, 1038. GC-MS m/z: 396 [M]+.
Benzyl acetate (Entry 8, Table 1), 1H NMR (300 MHz, CDCl3) δ 2.07 (s, 3H), 5.05 (s, 2H), 7.26 -7.36 (m, 5H). IR (cm−1): 3020, 1733, 1216, 756. GC-MS m/z: 150 [M]+.
Phenyl acetate (Entry 9, Table 1), 1H NMR (300 MHz, CDCl3) δ 2.21 (s, 3H), 7.0-7.4 (m, 5H). IR (cm−1): 1763, 1193, 748. GC-MS m/z: 136 [M]+.
4-Methoxyphenyl acetate (Entry 10, Table 1), 1H NMR (300 MHz, CDCl3) δ 2.1 (s, 3H), 3.8 (s, 3H), 6.70-7.0 (m, 4H). IR (cm−1): 3019, 2960, 1758, 1506, 1217, 1193, 755. GC-MS m/z: 166 [M]+.
4-Methylphenyl acetate (Entry 11, Table 1), 1H NMR (300 MHz, CDCl3) δ 2.01 (s, 3H), 2.25 (s, 3H), 6.92 (d, 2H), 7.04 (d, 2H). IR (cm−1): 3027, 2953, 1738, 1160, 754. GC-MS m/z: 150 [M]+.
1-Naphthyl acetate (Entry 12, Table 1), 1H NMR (300 MHz, CDCl3) δ 2.4 (s, 3H), 7.2-7.9 (m, 7H). IR (cm−1): 3061, 2924, 1767, 1368, 1200, 773. GC-MS m/z: 186 [M]+.
4-Chlorophenyl acetate (Entry 13, Table 1), 1H NMR (300 MHz, CDCl3) δ 1.98 (s, 3H), 7.0 (d, 2H), 7.25 (d, 2H); IR (cm−1): 1763, 1216, 1198, 756; GC-MS m/z: 170 [M]+.
2-Chlorophenyl acetate (Entry 14, Table 1), 1H NMR (300 MHz, CDCl3) δ 2.2 (s, 3H), 7.0 (d, 2H), 7.5 (d, 2H). IR (cm−1): 1763, 1216, 756. GC-MS m/z: 170 [M]+.
N-phenylacetamide (Entry 1, Table 2), 1H NMR (300 MHz, CDCl3) δ 2.19 (s, 3H), 7.16-7.11 (m, 1H), 7.28 (broad s, 1H), 7.36-7.30 (m, 2H), 7.55-7.52 (m, 2H). IR (cm−1): 3293, 1662, 1598, 1557, 1500, 1431, 1368, 1325, 1262, 1040, 1012, 962, 906, 750. GC-MS m/z: 135 [M]+.
N-(2-hydroxyphenyl)acetamide (Entry 2, Table 2), 1H NMR (300 MHz, CDCl3) δ 2.24 (s, 3H), 6.90 (m, 1H), 7.02 (m, 2H), 7.42 (m, 1H), 8.80(s, 1H),9.11 (bs, 1H). IR (cm−1): 3403, 3150, 1658, 1587, 1539, 1446, 1397, 1287, 1103, 1037, 891, 767. GC-MS m/z: 151[M]+.
N-(3-hydroxyphenyl)acetamide (Entry 3, Table 2), 1H NMR (300 MHz, CDCl3) δ 2.10 (s, 3H), 6.54 (d, 1H), 6.88 (d, 1H), 7.05 (t, 1H), 7.30 (s, 1H), 8.97(s, 1H), 9.30 (bs, 1H). IR (cm−1): 3300, 3100, 1653, 1600, 1571, 1520, 1441, 1380, 1254, 1110, 1050, 820, 760, 720. GC-MS m/z: 151[M]+.
N-(4-hydroxyphenyl)acetamide (Entry 4, Table 2), 1H NMR (300 MHz, CDCl3) δ 2.03 (s, 3H), 7.00 (d, 1H), 7.50 (d, 1H), 8.97(s, 1H), 9.40 (bs, 1H). IR (cm−1): 3326, 3164, 1652, 1611, 1565, 1507, 1442, 1372, 1327, 1259, 1228, 1173, 1108, 969, 837, 808, 715, 687. GC-MS m/z: 151[M]+.
N-benzylacetamide (Entry 5, Table 2), 1H NMR (300 MHz, CDCl3) δ 7.36-7.29 (m, 5H), 6.06 (bs, 1H), 4.43 (d, 2H), 2.03 (s, 3H). IR (cm−1): 3294, 1646, 1548, 1500, 1283. GC-MS m/z: 149 [M]+.
N-(pyridin-2-yl)acetamide (Entry 6, Table 2), 1H NMR (300 MHz, CDCl3) δ 2.19 (s, 3H), 7.03 (m,1H), 7.60 (t, 1H), 8.18 (d, 1H), 8.26 (d, 1H), 8. 50 (bs, 1H). IR (cm−1): 3388, 2923, 1685, 1434, 1303, 744. GC-MS m/z: 137 [M]+.
3. Results & Discussion
Acetylation of 1-octanol (1 mmol) with acetic anhydride (2 mmol) in presence of 15 wt% of carbon-SO3H catalyst was studied at 65°C to afford 1-octyl acetate in 96% yield in 30 min. However, the acetylation of 1-octanol with acetic anhydride at room temperature resulted <50% acetylated product even after 4 h. After the successful acetylation of 1-octanol with excellent yield to octyl acetate, the effect of catalyst loading for the acetylation of 1-octanol with acetic anhydride (1:2 mmol) to octyl acetate was studied by varying the catalyst dosage from 2 to 20wt% of alcohol (Table 3). The reaction was found to be slow at room temperature and hence the reaction temperature was increased to 65°C. At this temperature, with the increase of the catalyst loading from 2 to 15 wt% of alcohol decreased the reaction time substantially from 120 min to 30 min with conversions ranging from 65% to 96% (entries 1 - 4, Table 3). However, further increase of the catalyst loading to 20 wt% decreased the reaction time marginally from 30 min to 25 min with 98% conversion. The reactions were monitored by GC

Table 1. Acetylation of alcohols, phenols by employing carbon-SO3H catalysta.

Table 2. Acetylation of aromatic amines by employing carbon-SO3H catalysta.

Table 3. Effect of carbon-SO3H catalyst loading on the acetylation of 1-octanola.
using HP-1 capillary column. Based on this study, 15 wt% of the carbon catalyst was found to be optimum for the acetylation of 1-octanol with 96% conversion at 65˚C in 30 min.
In order to establish the effectiveness and the acceptability of the method in a wider context of synthetic organic chemistry, acetylation of various alcohols and phenols with electron donating and electron withdrawing groups were studied in presence of acetic anhydride under optimum conditions and the results are given in Table 1. The study revealed that primary and secondary alcohols (entries 1-5, Table 1) are acetylated with similar yields (95% - 96%) within 30 min, where as acetylation of ethoxy alcohol (entry 6, Table 1) and phytosterols (entry 7, Table 1) resulted the corresponding acetylated products in 95% yields in 45 and 60 min respectively. Acetylation of phenols was found to be slow when compared to aliphatic alcohols. Phenols with electron donating and electron withdrawing groups (entries 9 - 12 and 14, Table 1) were acetylated in 91% - 93% yields within 45 - 60 min and similar observation was made in case of benzyl alcohol also (entry 8, Table 1). However acetylation of 2-chlorophenol resulted 2-chlorophenyl acetate (75%, entry 13, Table 1) in less yields when compared to 4-chlorophenyl acetate (92% yield, entry 12, Table 1) due to steric effect.
In a similar fashion acetylation of aromatic amines also executed, and resulted the corresponding acylated products in good yields (92% - 97%, entries 1 - 6, Table 2) in 30 min. To examine the chemo selectivity of the present method, bi-functional substrates containing -NH2 and -OH groups were studied (entries 3-5, Table 2). Selective acetylation of the -NH2 group in the presence of the -OH group was observed at room temperature with 2 equivalent of acetic anhydride to give corresponding Nacetate product, and no O-acetate product was observed under these conditions. This might be due to more nucleophilicity of amines than phenols. The present protocol is excellent for the acetylation of alcohols (primary and secondary), phenols, amines, and bi-functional compounds containing -NH2 and -OH groups.
To check the reusability of the carbon catalyst, benzyl alcohol was employed for the acetylation for five cycles under the optimum conditions (Figure 1). After each cycle the catalyst was recovered by filtration, washed with methanol, dried in oven at 120°C for 1h and reused and the yields were found to be reduced marginally from 92 to 89% after 5th cycle.
4. Conclusion
In conclusion, we have demonstrated a simple, efficient and eco-friendly protocol for the solvent-free acetylation of alcohols, phenols and amines with acetic anhydride employing a novel glycerol-based -SO3H functionalized carbon as a solid acid catalyst. The low cost and simple preparation of the catalyst, and the easy procedure and work-up indicate that this catalyst is very attractive for this type of reactions. The yields are very good, and in addition, the carbon-based solid acid catalyst can be recovered by simple filtration for reuse without any pretreatment. The carbon-based solid acid can be used in place of sulfuric acid in the synthesis of organic compounds.

Figure 1. Recycling study of the carbon-SO3H catalyst for the acetylation of benzyl alcohol with acetic anhydride.
5. Acknowledgments
Katakam N. Gangadhar and Manneganti Vijay thanks to CSIR New Delhi for SRF.
NOTES