1. Introduction
Overactive bladder (OAB) is a common pathologic condition resulting from a neurogenic or non-neurogenic etiology that can be related to outflow obstruction and/or aging, but most cases are idiopathic [1]. The mechanisms of idiopathic OAB have become the focus of intense interest because of high prevalence and serious QOL impact of OAB [2]. The occurrence of OAB may be involved in myogenic and neurological factors, particularly, alternation of detrusor excitabilities and/or sensory afferent [3], but these processes are thought to be complex. Although many theories have been proposed to explain the development of OAB, its pathogenesis remains unclear. To elucidate the mechanism of OAB is required, and there is a clinical need for an effective drug to treat OAB. The ion channels such as calcium channels and potassium channels, play important roles in determining the functional properties and are obvious targets for treatment of overactive bladder [4]. Potassium channels play an important role for the membrane potential, and opening of the potassium channel leads to hyperpolarization of the membrane of smooth muscle cells [5]. It also reduces the opening probability of ion channels which are necessary for membrane depolarization [6]. In this way, they lead to relaxation of the muscles or inhibition of contractility [7]. Large conductance, voltageand Ca2+- activated K+ (BK) channels are widely distributed in a variety of tissues including bladder smooth muscles. BK channels respond to elevation in intracellular calcium and membrane potential depolarization, breaking excitability of smooth muscle. In the urinary bladder smooth muscle, BK channels have been demonstrated to play an important role in controlling membrane potential and excitability and to regulate contraction and relaxation [8]. In the absence of BK channel activity, bladder contraction and urinary frequency were enhanced in mice [9]. In addition, it was reported that the BK channel opener increased bladder capacity without affecting maximum bladder contraction pressure in accordance with decreasing afferent pelvic nerve activity [10]. Therefore, BK channels have attracted considerable interests as candidate targets for overactive bladder therapy [11]. BK channels are composed of 2 distinct integral subunits, α and β [12]. Only one type of α-subunit (KCNMA1) has been identified, whereas 4 putative β-subunit types (KCN-MB1-4) that are tissue-specifically distributed have been cloned [12,13]. In the present study, we investigated the expression pattern of BK channel subunit in the human urinary bladder. Moreover, we examined how its expression changes in association with bladder outlet obstruction (BOO), since BOO frequently causes overactive bladder. Because of possible involvement of bladder epithelium in sensory transduction, the mucosa and muscle layers were separately examined.
2. Materials and Methods
2.1. Patients and Bladder Sampling
Control bladder samples were obtained from 7 male patients without prostatic enlargement and lower urinary tract symptoms who underwent total cystectomy due to bladder carcinoma. BOO bladder samples came from 4 male patients who were performed suprapubic prostatectomy for benign hyperplasia of prostate (BPH). Tissue samples were mainly obtained from the anterior wall of the bladder. A mean age was 67.1 ± 12.7 years (ranging from 49 to 81) in controls and 73.8 ± 16.3 years (ranging from 65 to 93) in patients with BOO. Means ± standard deviations of International prostate symptoms score (IPSS) and prostate volume measured by ultrasound were 7.6 ± 4.2 (range 2 to 11) and 19.6 ± 9.7 ml (range 10.4 to 28.5) in control group, and t 24.5 ± 4.8 (range 17 to 30) and 62.9 ± 50.8 ml (range 47.3 to 155.7) in BOO group, respectively (Table 1). All samples were taken after obtaining informed patient consent. And the protocols of study were approved by the Ethical Committee in the University of Yamanashi.

Table 1. Characteristics of controls and patients with bladder outlet obstruction.
2.2. Tissue Preparation
About 5 mm in width tissue of bladder was excised and removed thesurrounding adipose and connective tissue. The mucosal layer of the bladder wall was peeled and cut away from the muscle layer, and each of mucosal and muscular specimens were processed immediately.
2.3. Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR)
Each mucosa or muscle tissue was homogenized in RNA stabilization solution (RNA later, Ambion; Austin, Texas, US) overnight at 4˚C. Total RNA was isolated from the mucosa or the muscle using the RNeasy Mini Kit (Qiagen, Hilden, Germany) and quantified by a spectrophotometer (GeneQuant; Biochrom, Cambridge, UK). The extracted RNA was reverse-transcribed using 1st Strand cDNA Synthesis Kit for RT-PCR (Roche, Basel, Switzerland) with random primer. Then, a quantitative real-time RT-PCR was performed with Smart Cycler System (Cepheid, Sunnyvale, CA, USA) using SYBR Green I (Molecular Probes, OR, USA) as the fluorogenic dye. The RT-PCR product (cDNA: 2 μl) was added into a 25 μl reaction system of Extaq R-PCR (Takara, Otsu, Japan) and each pair of specific primers, and subjected to 40 PCR cycles with a thermal program of 15 seconds at 95˚C for denaturation, 20 seconds at 55˚C for annealing and 20 seconds at 72˚C for extension. The primer sequences for the human BK channel subunits were in Table 2 (F, forward; R, reverse).
All primers and the sequences of products were confirmed using previously published sequences in the human genome database (GenBank Nos. NM 002247, NM 004137, NM 181361, NM 171830, NM 014505). The expression of BK channel subunit was normalized as the ratio to β-actin expression (×103) in each sample. Human renal segments and adrenal medulla in which the expression of BK subunits was identified, were used as positive controls [13-15]. Samples not treated with the reverse transcriptase were served as negative controls. Amplified PCR products were electrophoresed on 2% agarose gel and visualized with ethidium bromide to confirm the

Table 2. The primer sequences for the human BK channel subunits.
target band size. Some PCR products were purified and sequenced using an automated sequencing machine to identify the target gene.
2.4. Immunohistochemistry
Tissue pieces were mounted with tissue-TEK OCT compound (Sakura Finetek USA Inc., Torrance, CA, USA) and immediately frozen in liquid-nitrogen. For immunohistochemistry, 8 μm thick cryosections mounted on polylysine-coated slides were air-dried and fixed in acetone for 10min at 4˚C. Then, endogenous peroxidase activity and non-specific staining were blocked with 0.03% hydrogen peroxide for 10 min and blocking reagent X0909 (Dako, Glostrup, Denmark) for 20 min at room temperature. The sections were incubated for 24 hours at 4˚C with affinity-purified anti-BK α goat polyclonal antibodies (sc-14746; Santa Cruz Biotechnology, Inc., CA, US) at a dilution of 1:500. The slides were incubated with rabbit anti-goat immunoglobulins (HISTOFINE simple stain MAX-PO (G); Nichirei, Tokyo, Japan) for 30 min at room temperature. Each incubation step was followed by gently washing in Tris-buffered saline. The reaction products were developed with 3-amino-9-ethylcarbazole (AEC) solution for staining. Nuclei were counter stained with the use of hematoxyline, and the sections were coverslipped with Glycergel mounting medium (Dako). Negative controls were prepared in a similar manner, but consisted of omission of the primary antibodies.
3. Results
3.1. Quantitative Real-Time RT-PCR
RT-PCR analysis revealed the presence of α- and β1- subunit genes of BK channel not only in the muscle layer, but also in the mucosal layer (figure 1(A)). The expression levels of α1- and β1-subunit mRNAs in the muscle layer were significant higher than those in the mucosal layer (α: p = 0.0001, β1: p = 0.0001). A small amount of


(A) (B)
Figure 1. Expression of BK channel mRNA in the mucosa and muscle of human urinary bladder. (A) Representative PCR result of BK channel cDNA in agarose gel electrophoresis. Alpha-, β1-, β2-, β3-, and β4-subunits of BK channel and β-actin in mucosal and muscle layers are shown; (B) Quantitative analysis of BK channel expression using real-time RT-PCR. Alpha-, β1-, β2-, β3-, and β4-subunits of BK channel expression in mucosal or muscle layers were normalized as the ratio to b-actin expression in each sample. Asterisks indicate statistically significant differences in subunit expression between mucosal and muscle layers (*p = 0.0001; **p = 0.0001).
RT-PCR product of β4-subunit was identified in the mucosal layer, while β2-, β3-subunit genes were sub-stantially absent in both the mucosal and muscle layers (figure 1(b)). Statistical analyses were used by Welch’s t test.
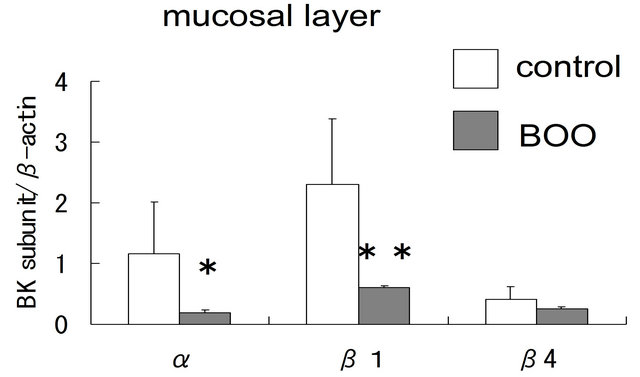
In the mucosal layer, the expression levels of α- and β1-subunit mRNA were significantly lower in BOO bladder than in controls (α; p = 0.03, β1; p = 0.02), but that of β4-subunit mRNA were not changed (p = 0.30) (figure 2(A)). Compared with controls, the expression of α-, and β1-subunit genes in the muscle layer was also significantly reduced in BOO bladder (α; p = 0.0003, β1; p = 0.0003) (figure 2(B)).
The expression level of α-, and β1-subunit genes detected in the mucosal layer were strongly correlated to storage symptom score of IPSS, (α; r = 0.8450, β1; r = 0.8457, respectively), so were in the muscle layer (α; r = 0.9340, β1; r = 0.9170) analyzed by Speaman’s rank correlation coefficient (Table 3).
Furthermore, significant relationships were noted between IPSS and α- and β1-subunit in the mucosal layer (α; r = 0.7970, β1; r = 0.8595), and those in the muscle

(A) (B)
Figure 2. Comparison of BK channel expression between control and obstructed bladders using real-time RT-PCR. (A) The expression of α-, β1- and β4-subunit genes in the mucosal layer of control and obstructed bladders; (B) The expression of α-, and b1-subunit genes in the muscle layer of control and obstructed bladders. Asterisks indicate statistically significant differences in subunit expression between control and obstructed bladders ((A): *p = 0.03; **p = 0.02; (B): *p = 0.003; **p = 0.003).

Table 3. Relationship between IPSS storage score and each BK channel subunit.
layer (α; r = 0.8957, β1; r = 0.9131) (Table 4).
3.2. Immunohistochemistry
The expression and localization of BK proteins were examined in the human urinary bladder. Immunohistochemical staining for BK α-subunit was localized in the muscle bundle and submucosal region, but there was little positive reaction in the epithelium (figure 3). In mucosal layer, the differences in staining density were unclear between controls and BOO bladders. On the other hand, the α-subunit immunoreactivity was more
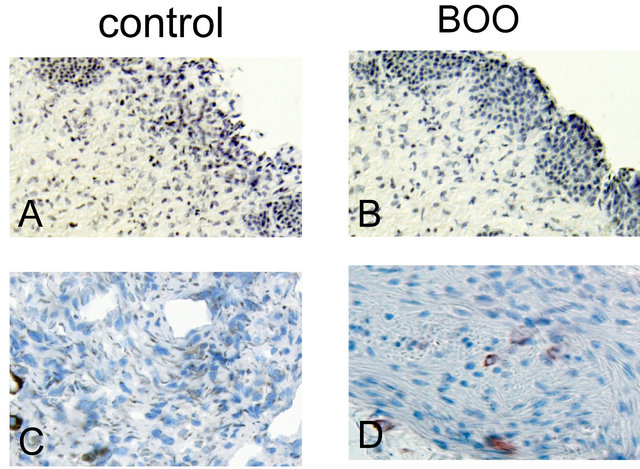
Figure 3. Immunohistochemical staining. Mucosal layers of the bladder (A and B) were reduced from ×200, and the detrusor muscle bundles (C and D) were reduced from ×400. Brown areas indicate positive reaction for BK a-subunit. In the bladder mucosa, the positive staining for BK channel was observed in the submucosal area, and its staining was a little weaker in the obstructed bladder (B) than in the conrol (A). In the muscle bundle, the positive staining was not remarkably different between the obstructed (D) and control (C) bladder.
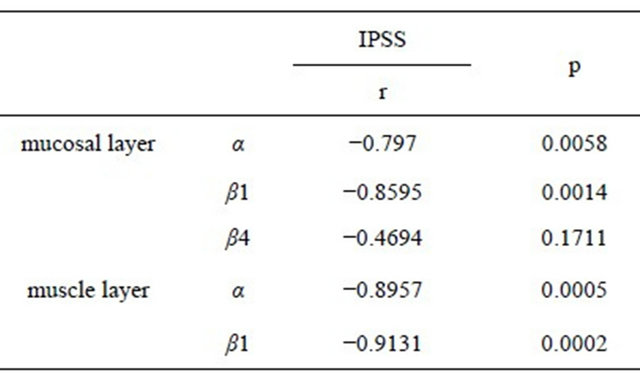
Table 4. Relationship between IPSS and each BK channel subunit.
densely observed in the muscle bundles of control bladders than in those of BOO bladders.
4. Discussion
The activation of K+ channels in many cell types including smooth muscles and neurons leads to membrane hyperpolarization and inhibition of action potential generation [11,13]. They are activated by several factors such as voltage, intracellular calcium, G-proteins and ATP [12]. One such class of K+ channels is calcium-activated K+ channels, which are classified into three broad categories according to their biophysical, molecular and pharmacological properties: large conductance (BK or MaxiK), intermediate conductance (IK), and small conductance (SK) channels [11]. BK channels were first studied in smooth muscle cells where they are the key players in setting the contractile tone [11,16]. However, they are also abundant in other tissues such as the brain, kidney and pancreas [17]. Because of their unique activation profile by both membrane depolarization and elevated intracellular Ca2+ levels, BK channels serve as ideal negative feedback regulator by decreasing voltage-dependent calcium entry via membrane hyperpolarization [18].
In urinary bladder smooth muscle, BK channels have been demonstrated to play an important role in controlling membrane potential and excitability [16], and to regulate contraction and relaxation [8]. In the human urinary bladder, we demonstrated that BK channels are expressed both in the muscle layer as well as in the mucosal layer by the use of genetic and histocytochemistrical approaches. Moreover, the expression patterns of subunit mRNAs in both tissues were elucidated. BK channels are composed of the pore-forming α-subunit and the regulatory β-subunit [12,13]. The present results showed that the BK channel is dominantly composed of α- and β1-subunits in both mucosal and muscle layers of human urinary bladder. However, a small amount of β4-subunit was noticed to express in the bladder, especially mucosal layer. Four different β-subunit is distributed in a tissue-specific manner [11,12]. It is known in the human tissues that β1-subunit mRNA is abundant in the smooth muscles, and β4-subunit is expressed mainly in the nervous system [17]. Thus, one possible reason for the expression of β4 subunit mRNA in mucosa tissue is that RT-PCR detected a small amount of β4 subunits expressed in subepithelial sensory nerves contaminating the mucosa samples. In fact, immunohistochemistry indicated the BK channels are located in submucosal region rather than epithelium. In both muscle and mucosal layers, the expression level of α- and β1-subunit mRNA remarkably decreased in BOO bladders compared with in controls. In immunohistochemistory using α-subunit antibody, the same finding was obtained in muscle layer. However, in submucosal region, the difference in immunohistochemical staining was unclear between controls and BOO bladders. The limited ability of conventional immunohistochemistry may be a reason for this detection failure.
The absence of functional BK channels in the detrusor significantly enhances basal and nerve-mediated bladder smooth muscle contractility, leading to bladder overactivity and urinary incontinence [9,19]. Additionally, the relaxation of bladder smooth muscle by β-adrenoceptor activation is mediated by BK channel opening [20]. Thus, the decreased expression of BK channels in BOO detrusor may lead to enhancing detrusor tone during urine storage. In addition to myogenic hypothesis for the OAB associated with BOO, the alterations of sensory afferent activity may be implicated in the underlying mechanisms [3,21]. The sensory transduction process seems to be more complex than originally thought. Recently, the bladder epithelium has shown to an important role in mechanosensory transduction by releasing neurotransmitters in response to various stimuli [22]. BOO may affect the transmitter release from epithelium [23]. However, in the present study, positive immunoreactivities for BK-α subunit were found in the submucosal region, but not in the bladder epithelium. Although we could not clearly identify what structures are stained in the submucosal region, subepithelial sensory nerve terminals are possible stained ones because of the expression of neuron-specific β4-subunit mRNA. Other attractive candidate is myofibroblasts (so-called interstitial cells), which are located beneath epithelium and might be implicated in afferent transduction mechanism [24]. BOO seems to increase the number of myofibroblasts [25]. Furthermore, the stretches resulting from local phasic activity in the bladder detrusor generate the bladder afferent discharges and correspond to urgency, suggesting the sensory function of motor activity [21,26]. Thus, the alteration of detrusor tone and/or excitability by decreased BK channel expression might affect the afferent activity.
5. Conclusion
BK channels, which are mainly composed of α- and β1-subunits, are expressed in both the mucosal and muscle layers of human urinary bladder. The expression level of each subunit in BOO bladder is lower than that in controls. Decreased expression of BK channel in BOO might be implicated in the mechanisms underlying the development of OAB.