1. Introduction
Muro Banded Iron-Formation (BIF) is one of the banded iron-formations (BIFs) associated with the Proterozoic metasedimentary “schist belts” in northern Nigeria (Figure 1). The BIF and associated metasediments form a range of elongate but rugged Muro Hills stretching for 13 km in a NE-SW direction. Two facies of the BIF, namely, oxide and carbonate facies [1] have been recognised with the former predominating and the latter being poorly developed and rather sporadic [2].
Since the discovery of the iron deposit by the Geological Survey of Nigeria in 1981, only reconnaissance studies have been done on its geological and structural setting as well as its geochemistry [3-6]. The present investigation is aimed at studying in some detail the geochemical characteristics of the iron-formation. Bulk chemical analysis of the iron-bearing rocks was carried out, and major and trace element contents of the BIF were compared with those of other Nigerian and Lake Superior-type and Algoma-type BIFs.
2. Geological Setting
2.1. Regional Geology
The Muro Banded Iron-Formation occurs in the Toto Schist Belt which is part of the Proterozoic supracrustal assemblage in the Nigerian basement complex [7]. The Nigerian basement is the southern part of Neoproterozoic Pan-African mobile belt situated east of the West African

Figure 1. Index map showing the location of the Muro district in the Pan-African province in relation to the West African and the Congo cratons.
craton. Most of the supracrustals forming the schist belts in Nigeria are exposed predominantly west of longitude 8˚. The age of the metasediments is uncertain as both Paleoproterozoic and Neoproterozoic ages have been obtained from them. In general, they bear imprints of polyphase deformation (D1 - D4) and metamorphism [8]. They consist essentially of pelitic and semi-pelitic schists and phyllites interlayered with minor to substantial quartzites, metaconglomerate, iron-formation, marble and amphibolites of tholeiitic affinity. The main deformation phase (D2) produced major folds such as the Muro antiform in the study area.
Both the basement and its supracrustal cover (the Schist Belts) have been intruded by a suite of Pan-African granites (Older Granites) with minor contact metamorphic and local migmatisation effects in the host rocks during the Pan African orogeny (ca 600 ± 150 Ma).
2.2. Local Geology of Muro Area
Four major lithological units which are interbanded have been mapped in the Muro area (Figure 2). These include marble, banded iron-formation, quartzites, and metasediments of pelitic to semi-pelitic composition. The various lithologies are folded into an overturned antiform trending NE-SW [4]. The marble occupies both the innermost core (M1) and flanks (M2) of the fold.
Successively following the former are bands of pelitic and semi-pelitic metasediments, quartzites and banded iron-formation. West of the Muro Hills, the metasediments are unconformably overlain by the Cretaceous Nupe Sandstone.
Bands of iron-formation (35 - 100 m wide/thick) occur between the outer zone marble and the quartzites. They outcrop in elongate ridges and irregular hills which take the arcuate form of the Muro Antiform (Figure 2) and can be traced for 20 km. Two generations of minor folds are exhibited in the iron-formation. These include tight, isoclinal, overturned folds having attenuated limbs with thickened or sharp crests and open folds with nearly horizontal axes. Both oxide and carbonate facies [1,9] occur but the former predominates with the latter being poorly developed. Occasionally bands of jasper are associated with the iron-formation.
The oxide facies iron-formation is a dark grey, finegrained, crystalline rock which displays banding on both centimetric and millimetric scales (i.e. mesoand microbanding). The banding consists of alternating light-coloured quartz-rich and darker-coloured iron oxide-rich layers. The mesobands range in width from about 1 cm to 2 cm (Figure 3) and the microbands from <0.5 - 2 mm (Figure 4).
The carbonate facies is a very hard, fine grained, massive rock made up of irregular brown siderite-rich and metachert-rich portions (Figure 5). It commonly occurs as rubble or vuggy boulders as found at west of Kuttiko in the southern area of the Muro Hills. Banding which is characteristic of the oxide facies is either non-existent or rare in this rock.
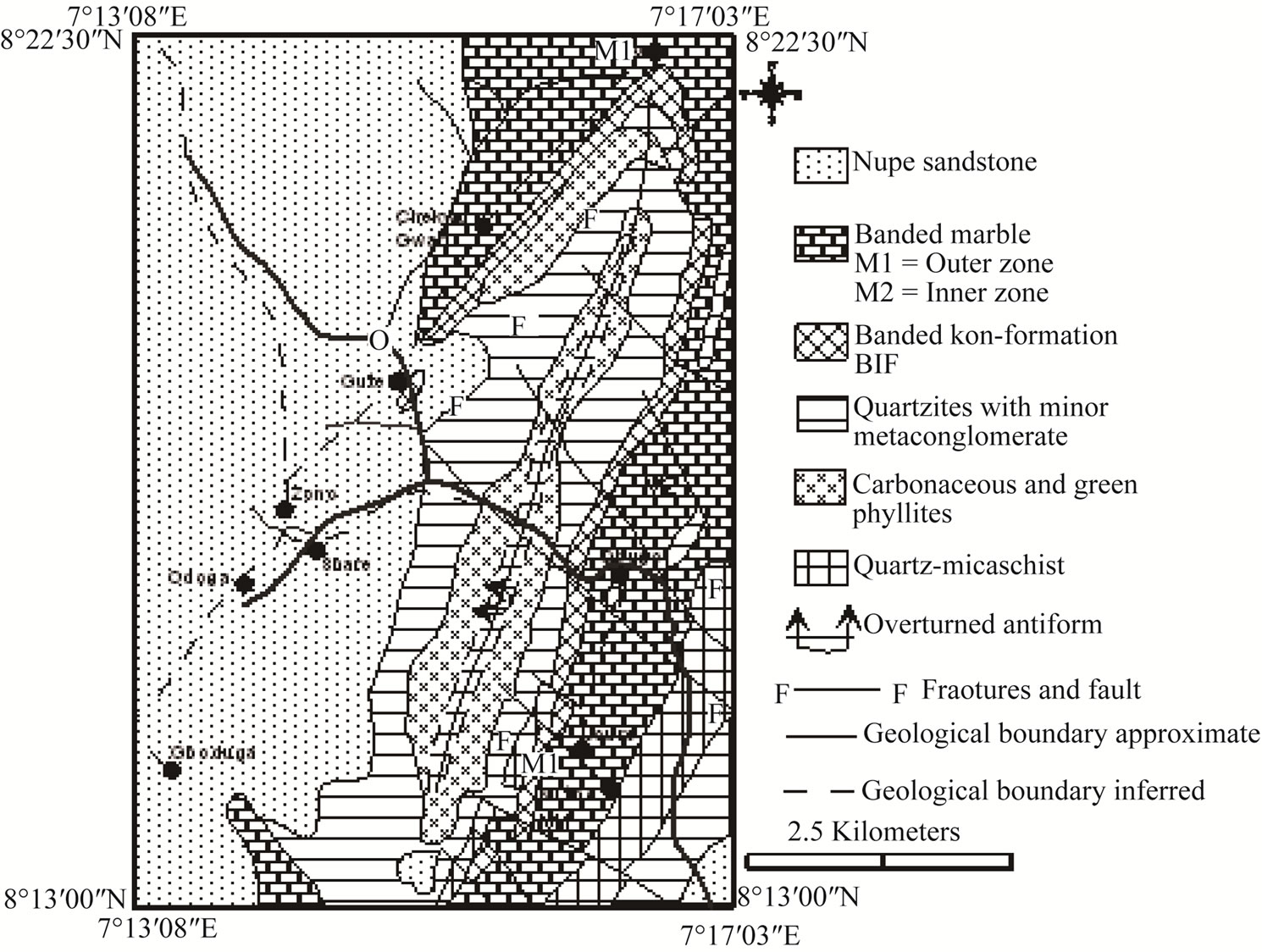
Figure 2. Geological map of Muro district, central Nigeria.

Figure 3. Photograph of Muro iron-formation oxide facies displaying dark iron-rich mesobands interbanded with lightcoloured quartz-rich ones.

Figure 4. Photomicrograph of polished section of the oxide facies of the Muro iron-formation displaying grey, hematite-rich microbands alternating with dark, quartz-rich ones. Reflected light; Magnification ×20.

Figure 5. Photomicrograph of carbonate facies iron-formation showing siderite grains oxidized to black iron oxides at grain boundaries. Transmitted light; crossed polars; Magnification ×25.
3. Analytical Methods
Representative samples of the two facies of the BIF were selected and analysed for both major and trace elements using a Phillips PW 1450 automatic sequential X-ray fluorescence (XRF) in the Department of Geological Sciences, McGill University, Montreal, Canada. Analyses were done on the fused beads for SiO2, TiO2, Al2O3, Fe2O3, MnO, MgO, CaO, Na2O K2O, P2O5, Ba, Cr, Ni, V and Zn. Other trace elements—Nb, Pb, Rb, Th, Sr, Cu, Co, U, Y and Zr—were analysed on pressed rock powder pellets by XRF spectrometry. The loss on ignition (LOI) of each rock powder was calculated after igniting to a constant weight.
4. Results and Discussion
4.1. Major Element Geochemistry
Geochemical analyses (Table 1) have revealed the essentially bimodal composition of the iron-formation with silica (SiO2) and total iron (as Fe2O3t) constituting over 85% of its components irrespective of the facies. Other components except the volatiles (LOI) occur in very small amounts. An antithetic relationship exists between the SiO2 and total Fe2O3, which is reflected in the strong negative correlation between the two components (Figure 6) and in the empirical inverse distribution of quartz and iron minerals in the iron-formation. In this respect Muro iron-formation is similar to iron-formations in other parts of the world [10-12]. A plot of the Muro samples in the compositional diagram after [10] shows that they lie in the Precambrian BIF field (Figure 7), thus indicating that the Muro BIF is geochemically similar to other Precambrian iron-formations.
Silica (SiO2) content in the oxide facies ranges from 50.33% - 64.50% with an average of 56.53%. Higher values obtained from the carbonate facies samples could be due to highly irregular distribution of the metachert and siderite and the difficulty in obtaining representative samples on the field. However, the generally high silica contents in all the BIF samples are attributable largely to the high metachert or quartz constituents of the iron-formation.
TiO2 content (0.01% - 0.05%) is very low with essentially uniform distribution as most samples are in the range of 0.01% - 0.02 % (Table 1). Alumina content is low, ranging from 0.01% - 0.54% with an average of 0.28% for either the oxide or carbonate facies. In spite of the low concentration of alumina, it exhibits a weak positive correlation with TiO2 (Figure 8). This is probably inherited from the common association of Ti and Al in clayey materials derived from residual weathering products. Both Al and Ti are hydrolysate elements [13] which are transported together by rivers to the basin of deposition [14]. The presence of clay material in the iron-formation of which alumina is an index indicates an initial clastic dilution in the basin of deposition.
Total iron as Fe2O3 ranges from 33.15% to 48.08%, with an average of 41.69%, in the oxide facies. The iron content of the carbonate facies is extremely variable and its total iron content (Fe2O3t) ranges from 3.68% - 20.66%. The relatively low values of iron in this facies are attributable to the same factors that accounted for its
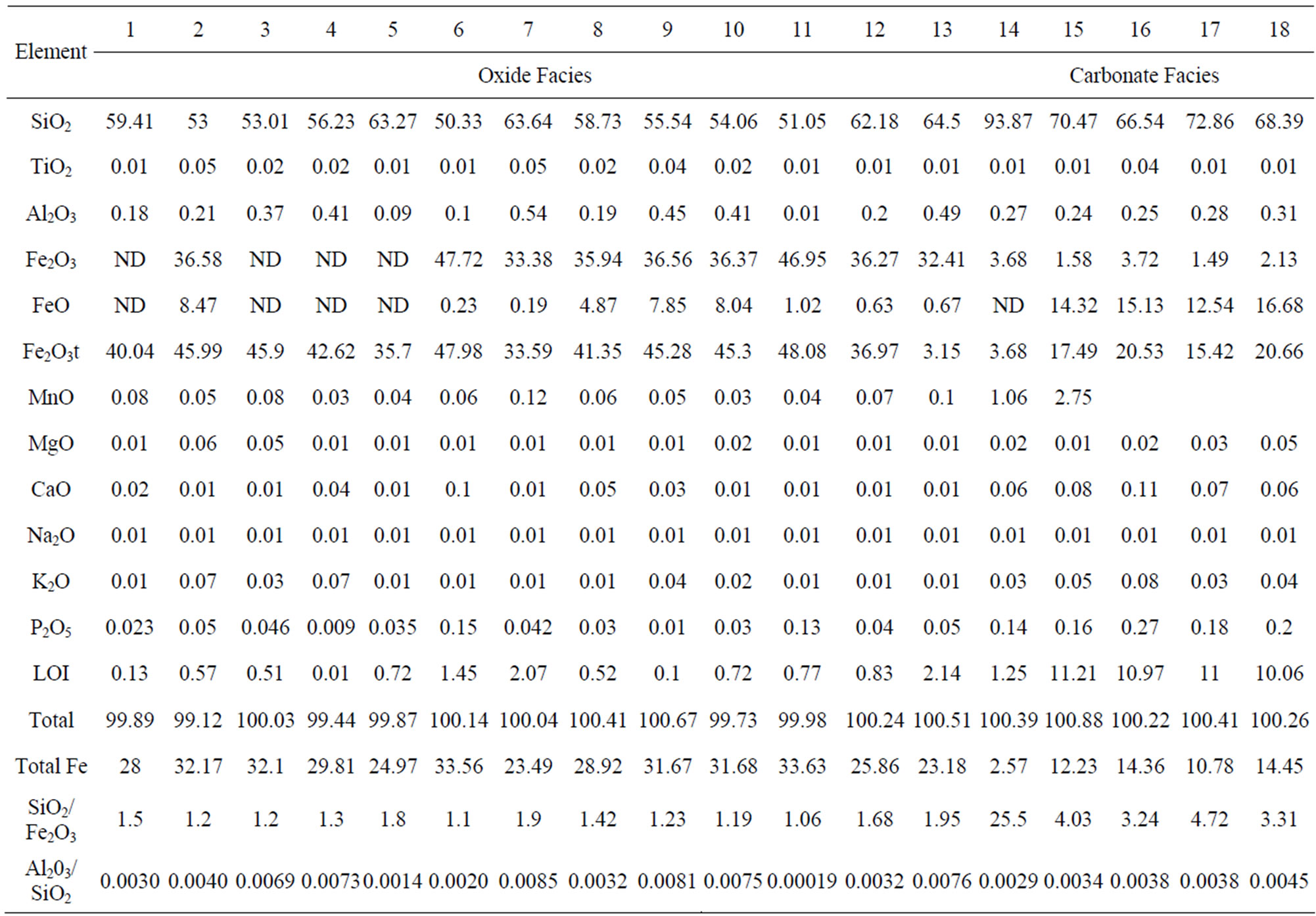
Table 1. Major element concentrations of the oxide facies (1 - 13) and the carbonate facies (14 - 18) of the muro banded iron-formation.
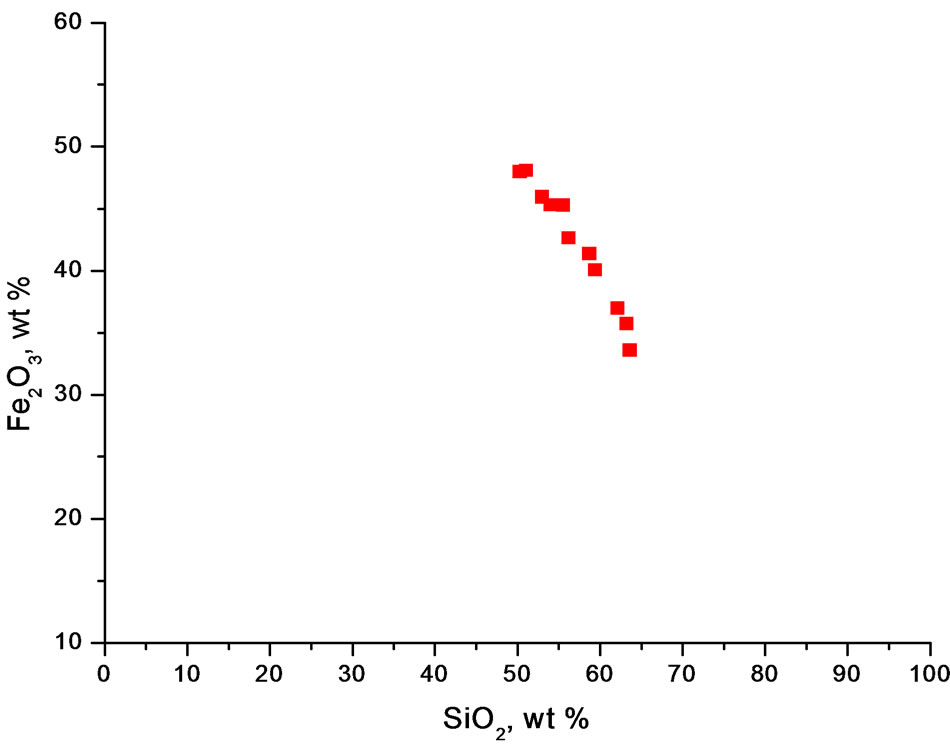
Figure 6. Plot of SiO2 against Fe2O3 for the Muro oxide facies showing a negative correlation between silicon and iron.
very high SiO2 values as explained above. Chemical analyses (Table 1) have revealed high Fe2O3 and low FeO values in the oxide facies in consonance with the preponderant hematite and subordinate magnetite in the
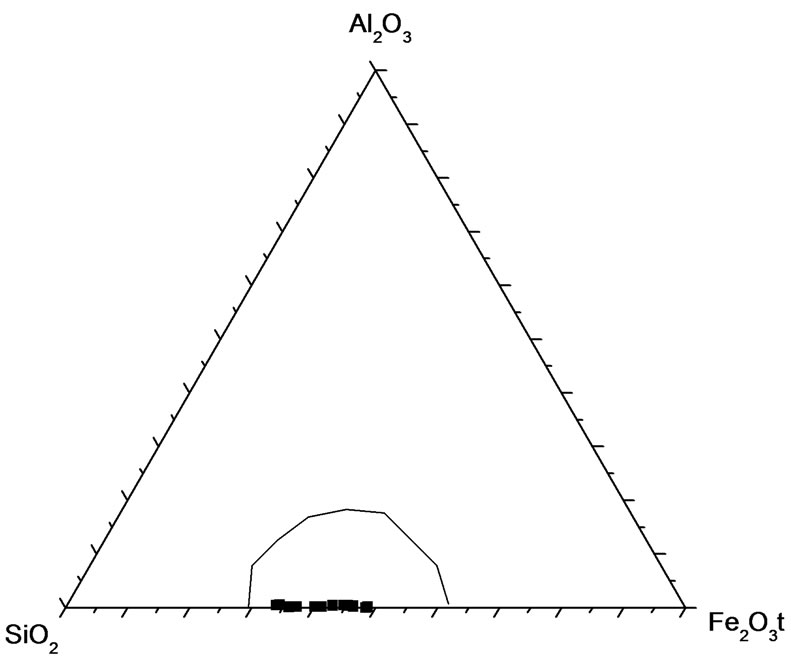
Figure 7. Al2O3-SiO2-Fe2O3t triangular diagram showing the field of Precambrian Banded iron-formations (BIF) after Govett (1966) and the plots of the Muro oxide facies samples correlation between the two elements.
rock. Similarly, the carbonate facies contains relatively high FeO and low Fe2O3 components, thus reflecting the presence of siderite as the main mineral in that facies.
Manganese content is low (mean 0.06%) and is distributed somewhat uniformly in the oxide facies but it is
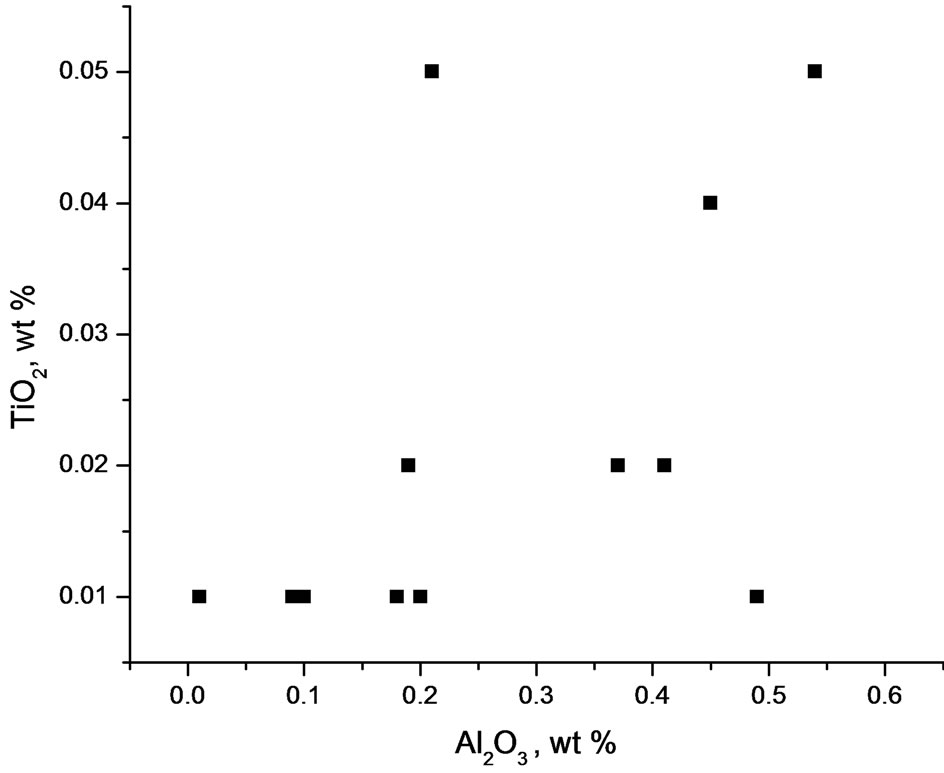
Figure 8. Plot of Al2O3 against TiO2 for the Muro oxide facies showing some positive correlation albeit with some scatter.
much higher (mean 2.23%) in the carbonate facies.
Very low concentrations of magnesium and calcium are present in the iron-formation irrespective of its facies. MgO content ranges from 0.01% - 0.06% and CaO content from 0.01% - 0.11%. The low concentrations of the two elements especially when combined with the low volatile contents (LOI) of the oxide facies indicate paucity of carbonate and silicate minerals in the facies [15], which is also confirmed from petrographic studies.
The alkali metal contents (Na2O and K2O) are very low with most samples having values of 0.01% especially in the case of sodium. This also reflects the rarity of silicate minerals in the iron deposit.
Phosphorous content (P2O5) is low varying from 0.01% - 0.27% in the iron-formation. Its distribution tends to be uniform in the oxide facies which also displays much lower concentration (average 0.05%) than the carbonate facies (average 0.19%).
Compared with the data from [16] and [12] the mean contents of Fe2O3, TiO2, MnO, MgO, CaO, Na2O, K2O, P2O5 and Al2O3 in the Muro oxide facies are similar to those of the Lake Superior and the Algoma oxide facies (Figure 9, Table 2). However, the Muro oxide facies has a slightly higher SiO2 content relative to the others (Figure 9).
4.2. Trace Element Geochemistry
The trace elements presented in Table 3 are expressed in averages and ranges in Table 4 for comparison with the average trace element contents of other iron-formations. The low values of these elements in the Muro Iron-Formation are similar to their values in Precambrian ironformations in other parts of the world [12,15,17-19].
Its oxide facies (Table 3, analyses 1 - 13) appears to be more impoverished in trace elements than the carbonate facies (analyses 14 - 18) which contains relatively high concentrations of Ba, Cu, Ni, Mn, Co, and V compared with even the Lake Superior-type and Algoma-type BIFs (Table 4). Occurring in particularly low concentrations (lower than the detection limits of the analytical instrument) are Ni, Rb, Y and Zn in the oxide facies and Rb and Zn in the carbonate facies (Table 3).
In general, the level of trace element concentrations in the Muro oxide facies is less than those of its Lake Superior-type and Algoma-type BIFs (Table 4, Figure 10).
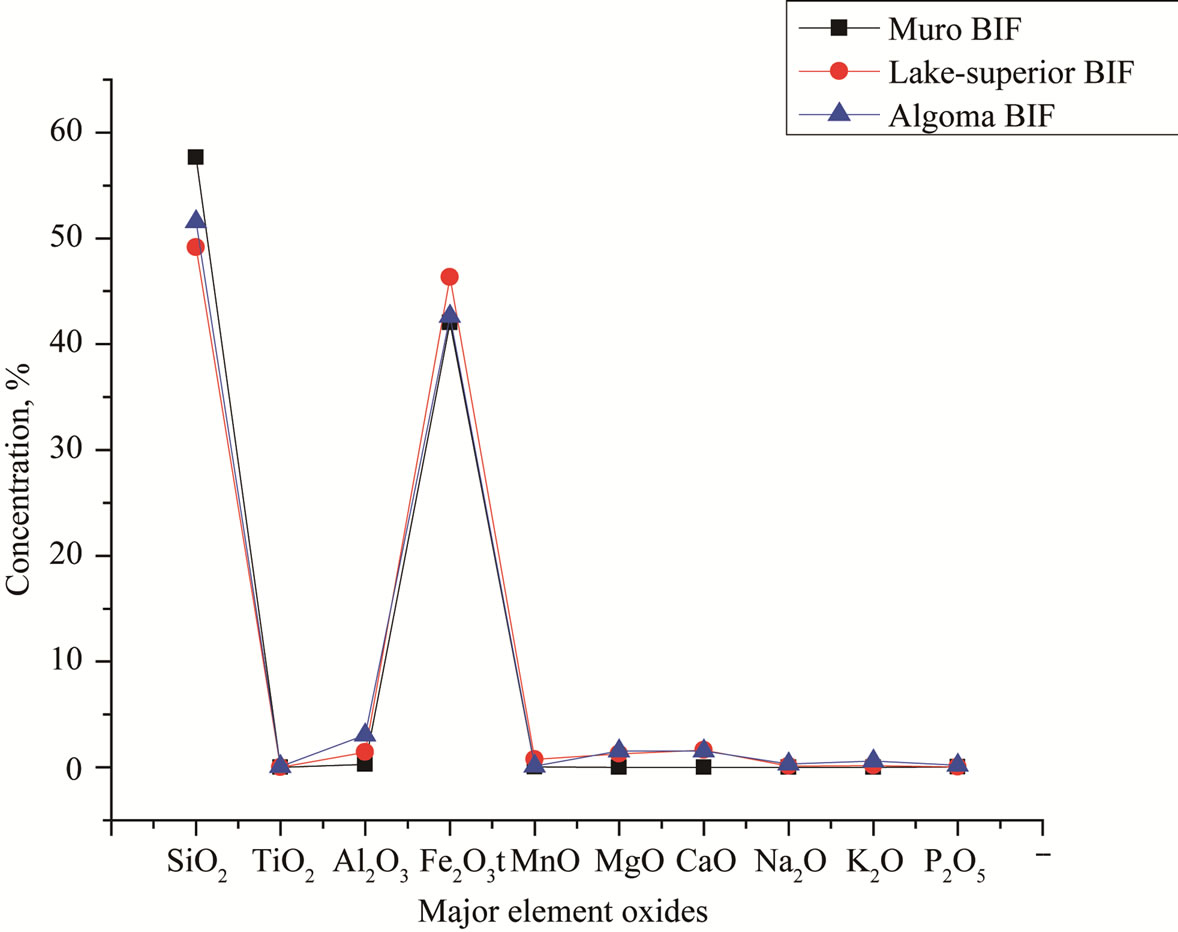
Figure 9. Spider diagram comparing the major element composition of Muro BIF with those of Lake Superior-type and Algoma-type BIFs.
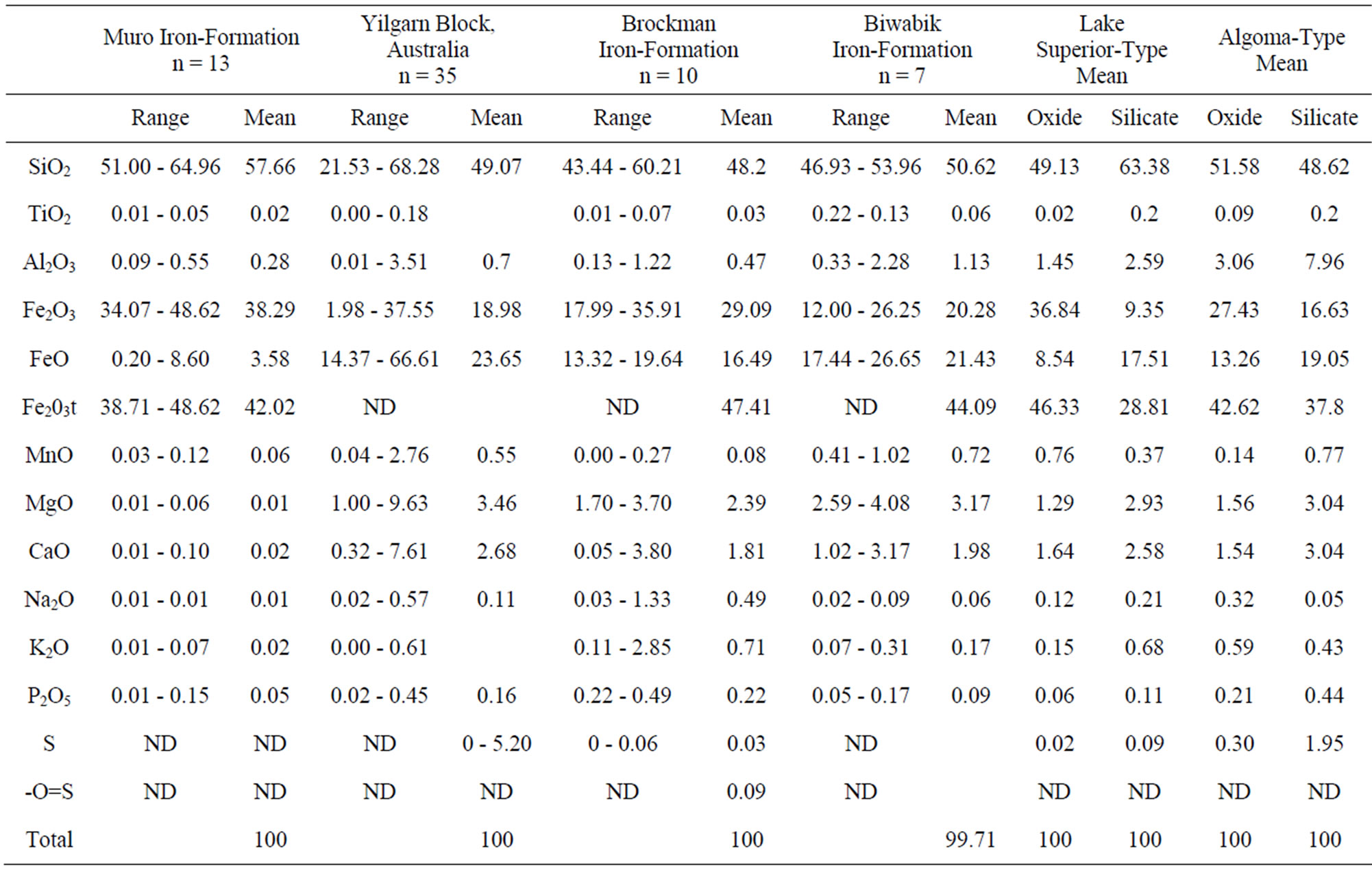
Table 2. Major element summary statistics (range, mean and number of samples, n) of Muro iron-formation and some other iron-formations from different parts of the world.
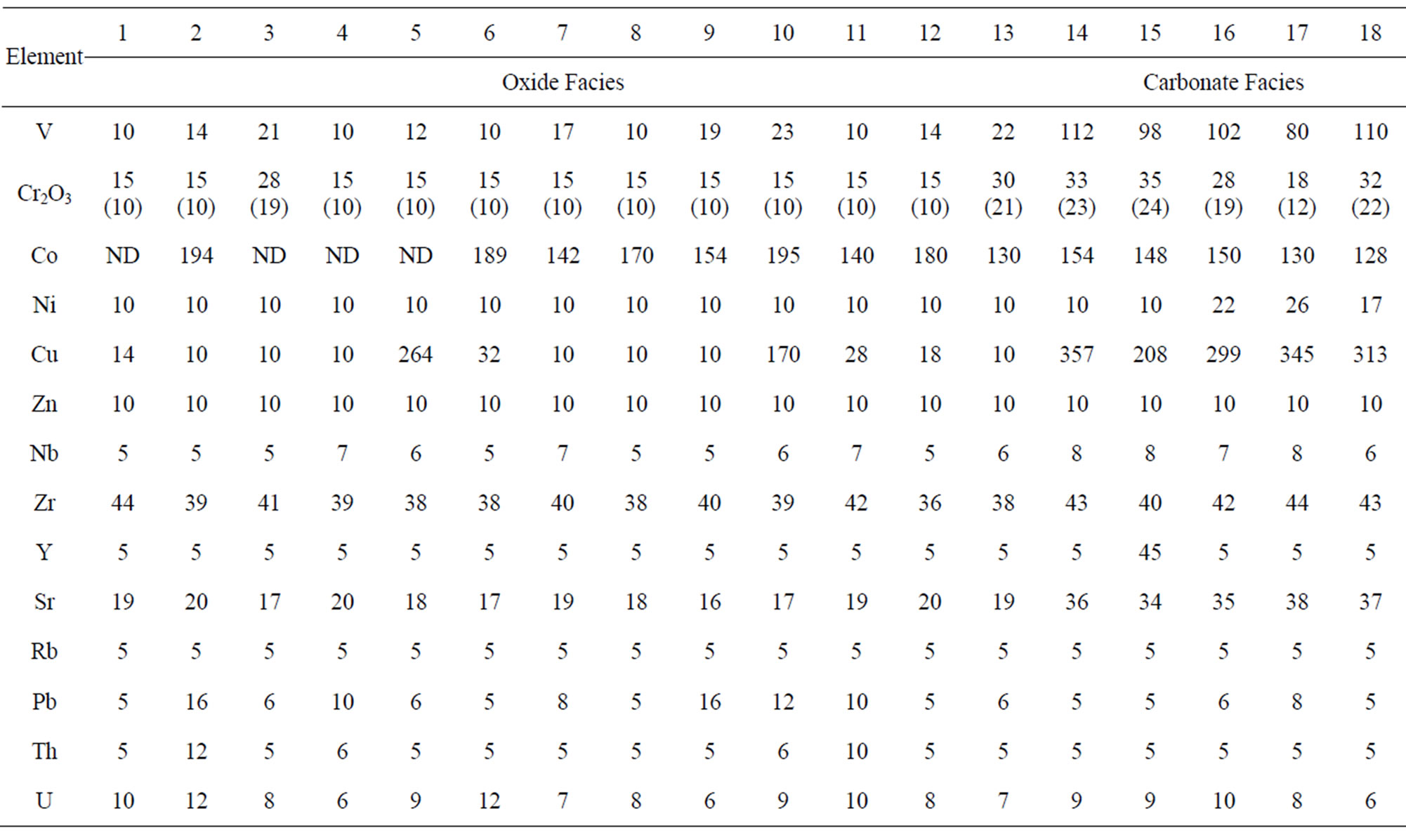
Table 3. Trace element concentrations of the oxide facies (1 - 13) and the carbonate facies (14 - 18) of the muro banded iron-formation.
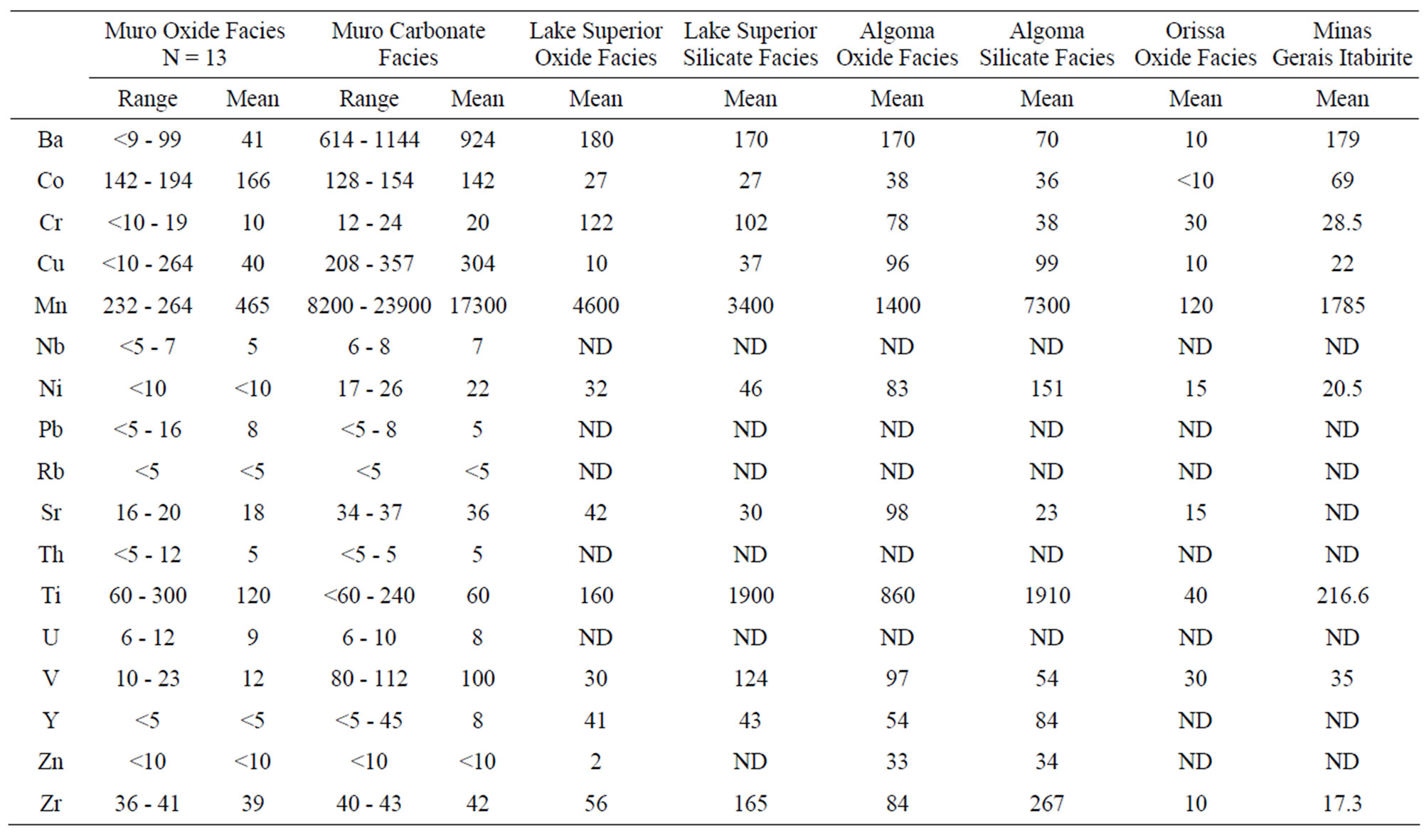
Table 4. Trace element summary statistics (range, mean and number of samples, n) of Muro Iron-Formation and mean of some other iron-formations from different parts of the world.
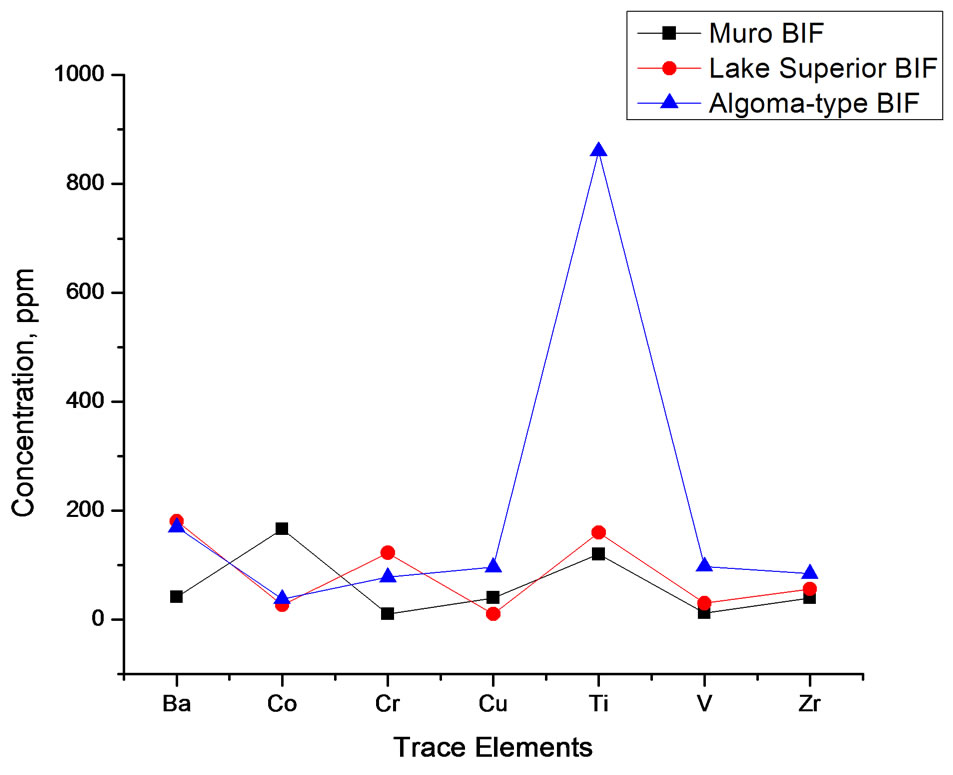
Figure 10. Spider diagram comparing the trace element composition of Muro BIF and those of Lake Superior-type and Algoma-type BIFs.
These differences in the trace element concentrations may be attributed to the level of detrital material (clay constituents) in the original iron-formation. The Al2O3 content, which is a measure of the detrital dilution of iron-formations [20], is less in the Muro oxide facies (0.28%) than in the Lake Superior oxide facies (3.06%) (Table 2). The geochemistry of the various trace elements is summarised as follows.
Chromium and Niobium: Chromium concentration is low being in the range of 10 - 19 ppm in the oxide facies and 12 - 24 ppm in the carbonate facies. Compared with those of the Lake Superior-type and the Algoma-type iron-formations (Table 4) the Cr concentration is particularly low but it is closer in value to those of the Minas Gerais itabirite and the Orissa BIF. Such a low Cr concentration has been reported in Swedish iron ores and BIF deposited as oxidates in sedimentary environments [17,21]. Niobium concentration is low (5 - 8 ppm) but its distrbution tends to be uniform in both the oxide and carbonate facies. Because this element has not been determined in most iron-formations it is impossible to compare its level of concentration in the Muro oxide facies with those of other iron-formations.
Vanadium: Vanadium distribution pattern in the Muro iron-formation is facies dependent. The vanadium content of the oxide facies is low (average 12 ppm) while that of the carbonate facies is comparatively higher (average 100 ppm). A similar relationship exists in the Lake Superior-type BIF (Table 4).
Yttrium: Yttrium concentration is very low. If one sample that gave an anomalous value of 45 ppm is ignored, all the remaining samples contain little (less than 5 ppm) or no yttrium at all.
Zirconium, Uranium and Thorium: Zirconium content is relatively low and its distribution is somewhat uniform in both the oxide and carbonate facies with its concentration ranging from 36 - 43 ppm. The average Zr contents of the Muro oxide-facies (39 ppm) and carbonate-facies (42 ppm) are less than those of their corresponding facies in the Lake Superior-type and Algomatype iron-formations. Since the distribution of this element is attributed to provenance rather than to sorting of sediments [17], its low level of concentration therefore indicates that the iron-formation is derived from low Zr source materials. This possibility excludes igneous derivation of the iron-formation but favours a sedimentary origin.
Uranium: Uranium concentration is very low (ranging from 6 - 12 ppm) with a uniform distribution pattern as both oxide and carbonate facies display similar average U contents (8 and 9 ppm, respectively).
Thorium: Thorium occurs in generally very low concentrations (5 - 12 ppm) with most samples being below the detection limit (5 ppm).
Cobalt and Nickel: Cobalt content of the Muro ironformation is high, varying from 128 - 194 ppm, and it contrasts greatly with low contents of the Lake Superiortype, Algoma-type and Orissa BIF as well as the Minas Gerais itabirite (Table 4). In the Muro iron-formation the cobalt concentration of the oxide facies (142 - 194 ppm) is higher than that of the carbonate facies (128 - 154 ppm). Nickel concentration is generally very low in the Muro iron-formation, ranging from <10 - 26 ppm. It is particularly low in the oxide facies (<10 ppm).
Copper, Lead and Zinc: The Muro iron-formation is relatively enriched in copper but is impoverished in lead and zinc. Cu distribution tends to be facies dependent with lower concentration in the oxide facies (average 40 ppm) compared with its higher content in the carbonate facies (average 304 ppm). A similar relationship has been found in the Isua BIF in Greenland [22].
Barium, Strontium and Rubidium: Both barium and strontium exhibit a similar distribution pattern. The concentrations of both elements are relatively high in the carbonate facies and are comparatively much less in the oxide facies (Table 3). The sympathetic behaviour of the two elements is attributable to the fact that Sr associates with Ba and Ca in nature [17]. Sr content is generally low in the Muro iron-formation being in the range of 16 - 37 ppm. The average Sr contents of both the oxide and carbonate facies compare favourably with those of the Lake Superior-type BIF but are appreciably less than those of the Algoma-type BIF (Table 3). On the other hand, the Ba content of the carbonate facies is very high (614 - 1144 ppm) with an average of 924 ppm while those of the oxide facies is not only low but highly variable (9 - 93 ppm). Rubidium is strongly depleted in the iron-formation, its value being below the detection limit (5 ppm) of the analytical equipment Generally, the trace element concentrations of the Muro oxide facies is similar to those of the Lake Superior oxide facies except for the much lower contents of Ba and Cr, and much higher contents of Co in the Muro BIF (Figure 10). On the other hand, the trace element contents of the Muro oxide facies differ from those of the Algoma oxide facies (Figure 10). The Muro BIF has significantly lower contents of Ba, Cr, Cu, Ti, V and Zr compared to the Algoma-type BIF.
5. Mineral Chemistry
Electron microprobe analysis of hematite in the oxide facies of Muro Iron Formation is presented while that of the carbonate mineral in the carbonate facies is presented. The hematite contains only minor amounts of MnO, Al2O3 and SiO2, while the carbonate is dominantly rhodochrosite with minor amounts of magnesia and siderite. Both analyses reveal the minor importance of manganese in the iron formation [cf 23].
6. Summary and Conclusions
The major and trace element distribution patterns of the Muro BIF are essentially similar to those of the Lake Superior-type BIFs as described by [12,15,17]. The observed trace element distribution and abundance patterns in the iron-formation also lend support to the shallow water sedimentary deposition of the iron-formation. Lower concentrations of Cr, Ti, Ni and Zr, (which have a strong igneous affinity) in the Muro Iron-Formation than in the Algoma analogue indicate a sedimentary origin of the iron-formation. Paucity of trace elements especially of Ba, Co, Ca, Mn, Ni and Pb in the iron-formation suggests shallow water deposition [15,17]. According to [17] very low concentrations of V, Cr, Zr, Ni and Cu in BIF indicate low temperature precipitation under sedimentary conditions. The Muro BIF contains particularly low contents of these elements (see Table 3), thus supporting the low temperature deposition of the iron-formation in a sedimentary environment.
The weak positive correlation of TiO2 with Al2O3 as well as their very low concentrations in the Muro BIF suggests only very minor terrigeneous contribution to its source materials (silica and iron) [24,25]. This observation is supported by the scarcity of silicate minerals in the mineral assemblage. Silica was deposited as chert as revealed by the occurrence of jasper and metachert in the iron-formation. The presence in the jasper of siliceous spheroidal structures which are ascribed to biogenic origin [26] suggests some biological contribution to the silica content of the BIF.
Muro Iron-Formation, which is made up of predominant oxide facies and subordinate carbonate facies, is of the Lake Superior-type. This is demonstrated by its similarity in both lithological association and geochemistry to the Lake Superior-type iron-formations. These similarities suggest common depositional environments and source materials.
7. Acknowledgements
We wish to express our profound gratitude to Professor Bob Martins of the Department of Geological Sciences, McGill University, Montreal, Canada for permitting the analysis of the samples to be done in the geochemical laboratories of his department. We are also grateful to colleagues in the Nigerian Geological Survey Agency and the Obafemi Awolowo University, Ile-Ife, Nigeria for their logistic support. The study was largely funded by the Federal University of Technology, Akure through the University Research Grant number URG 87-88/03.
NOTES