1. INTRODUCTION
The use of plants as source of remedies for the treatment of many diseases dated back to prehistory; and many populations have this old tradition. Despite the remarkable progress in synthetic organic chemistry in the twentieth century, over 25% of prescribed medicines in industrialized countries derived directly or indirectly from plants [1]. However, plants used in traditional medicine are still understudied, particularly in clinical microbial ogy. It is reported that most antibiotics derived from microorganisms, and one to three antibiotics are launched every year [2].
In addition, many people, principally in the developed countries are interested in having more autonomy over their medical care, so self-medication is commonplace [3].
Highly hydroxylated phenolic compounds present in the extractive fraction of several plant materials. Polyphenols in plants include hydroxycoumarins, hydroxylcinnamate derivatives, flavanols, flavonols, flavanones, flavones, anthocyanins, proanthocyanidins (tannins), hydroxystilbenes, aurones, etc. Polyphenols are well documented to have microbicide activities against a huge number of pathogenic bacteria [4].
The evaluation of the antioxidant activities of polyphenols from ethnomedicinal plants may also be necessary because they are among desired medicinal properties of plants due to their nutraceutical effects [5]. Antioxidants are compounds that can delay or inhibit the oxidation of lipids or other molecules by inhibiting the initiation or propagation of oxidizing chain reactions. The antioxidant activity of polyphenols is mainly due to their redox properties, which can play an important role in adsorbing and neutralizing free radicals, quenching oxygen, or decomposing peroxides.
Antioxidant activities of polyphenols have been suggested to exert a beneficial pharmacological effect on neurological disorders on the basis of in vitro observations [6]. Polyphenolic compounds in plant, including the catechins, exert anticarcinogenic, antimutagenic and cardioprotective effects linked to their free radical scavenging [7]. They are reported to be chemopreventive agents by lowering cholesterol and roughly limit cell damage [8]. In addition to their individual effects, antioxidants interact in synergistic ways and have sparing effect in which one may protect another against oxidative destruction. These justify the overwhelming interest in finding naturally occurring antioxidants for use in foods or medicinal materials to replace synthetic antioxidants [7].
The present work aims at studying the antibactérial activity and antioxidant rough extracts of an aromatic plant, Hertia cheirifolia which belongs to the family of Asteraceae.
The goals of this study is to evaluate the antioxidant and anti-ridicalizing activity using whitening test of the β-Carotene; and DPPH test; also to evaluate the antibacterial activity by the method of diffusion in medium gelose of the rough extracts of Hertia cheirifolia. This evaluation is connected to the phenolic contents of these extracts.
2. MATERIALS AND METHODS
2.1. Plants Materials
Our plant Hertia cheirifolia was collected from the mountains of Aures of Batna.
2.2. Extraction Procedures
Plants were dried in the shade to ambient temperature until total dehydration. Dried leaves parts were blended into fine powder and stored in the dark at a dry place. 50 g of the powdered leaves of plants were extracted in the 500 ml of ether de petrol, 500 ml of dichloromethane, 500 ml of methanol, and 500 ml of water respectively for 24 h under agitation at room temperature of 23˚C for 24 h. The extracts were concentrated by rotary evaporation (Heidolph) and the yield of extraction was determined. All the dried extracts were preserved in the refrigerator until further use. Plant extracts were dissolved in the methanol before use in the antimicrobial assay.
2.3. Determination of Total Phenolic Compounds
Total phenolic compounds from lyophilized samples were quantified using Folin-Ciocalteu’s method [9], 25 μl of Folin-Ciocalteu’s reagent (50%, v/v) was added to 10 μl of 1 mg/ml (w/v) of lyophilized plant extract dissolved in water. After 5 min incubation at room temperature, 25 μl of 20% (w/v) sodium carbonate and distilled water were added to a final volume of 200 μl per well. Blanks were prepared by replacing the reagent by water to correct for interfering compounds. After 30 min of incubation, the absorbencies were read at 760 nm using a spectrophotometer.
All the assays were carried out at least in duplicate. Gallic acid was used as standard and results were expressed as gallic acid equivalent per gram of lyophilized sample.
2.4. Determination of Total Flavonoids Compounds
Aluminum chloride colorimetric method was used for falconoid determination according to [10], each shrub extract (500 l) in methanol was separately mixed with 1.5 ml of methanol, 0.1 ml of 10% aluminum chloride, 0.1 ml of 1 M potassium acetate and 2.8 ml of distilled water. The prepared solution was left to stay for 30 min at room temperature, before being submitted to the absorbance measurements. The absorbance of the reaction mixture was measured at 415 nm using a spectrophotometer.
All the assays were carried out at least in duplicate. Quercetin was used as standard and results were expressed as quercetin equivalent per gram of lyophilized sample.
2.5. Determination of Antioxidant Activity
2.5.1. Evaluation of Antioxidant Activity Beta-Carotene Bleaching Test
A solution of -carotene/linoleic acid was prepared as follows: First, 0.5 mg of -carotene was dissolved in 1 ml of chloroform (HPLC grade), and then 25 l of linoleic acid and 200 mg of Tween 60 were added. The chloroform was subsequently evaporated using a vacuum evaporator. Next, 100 ml of distilled water saturated with oxygen (30 minutes at 100 ml/minute) was added with vigorous shaking.
Aliquots (2.5 ml) of this reaction mixture were transferred to test tubes, and 350 ul portions of the extracts (2 g/l in methanol) were added before incubating for 48 hours at room temperature. The same procedure was repeated with tocopherol at the same concentration and a blank containing only 350 l of ethanol. After the incubation period, the absorbance of the mixtures was measured at 490 nm. The antioxidant capacities of the samples were compared with those of tocopherol and the blank [11,12].
2.5.2. Determination of Antioxidant Activity Using the Free Radical Scavenging Activity (DPPH)
The method described by Tepe et al (2005) was used with slight modification, the different extracts and controls (ascorbic acid and tocopherol “antioxidants reference”; quercetin and gallic acid “pure substances”).
DPPH solution was prepared by dissolving 3 mg of DPPH in 100 ml of methanol, 50 ul of solution samples and controls are added to 2 ml of DPPH solution after incubation for 30 min in darkness at room temperature, absorbances were measured at 517 nm against the blank. The antioxidant activity is estimated using the following equation:

2.6. Antibacterial Activity
References bacterial strains were tested: Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, Staphylococcus aureus ATCC 25923. The bacteria were cultivated nutrient agar (NA) at 37˚C ± 0.2˚C. Susceptibility of the tested organism to the extracts was determined by employing the disc-diffusion method [13]. Sterile filter paper discs of 9 mm diameter impregnated with 40 μl of each test plant extract, were placed on the surface of the inoculated agar plates. The extracts were taken with Dimethyl sulfoxide (DMSO). The antibacterial screening was carried out with different concentrations for each extract (1 g/ml, 0.5 g/ml and 0.25 g/ml and 0.125 g/ml).
Controls soaked in DMSO only were performed. The Petri dishes were incubated for 24 h at 37˚C.
The antibacterial activity was determined by measureing with a rule the diameter of the zone of inhibition, determined by the different concentrations of various extracts around the discs.
2.7. Statistical Analysis
All tests were performed in triplicate. Results are presented as mean ± standard deviation of two or three independent determinations. All statistical analyses were carried out by Graphpad prism 5 using analysis of variance (ANOVA) and differences among the means were determined for significance at p ≤ 0.05 using least significant.
3. RESULTS AND DISCUSSION
3.1. Total Phenolic and Flavonoids Compounds
The results of the proportioning of total polyphenols reveal that EMe, EAq and EDm are rich in phenolic compounds (30.33 ± 2.82 μg EAG/mg of extract 25.92 ± 7.19 μg EAG/mg of extract and 21.25 ± 1.76 μg EAG/mg of extract respectively, while EEp contains (11.50 ± 4.00 μg EAG/mg of extract).
The quantitative estimate of the total flavonoïdes shows that EMe (8.98 ± 0.23) μg EQ/mg of extract and EAq (6.78 ± 0.22) μg EQ/mg of extract are rich in flavonoïdes thereafter comes EDm (4.25 ± 0.12) μg EQ/mg of extract and EEp (2.09 ± 0.00) μg EQ/mg of extract.
The estimation of these results makes it possible to put a significant linear correlation between the content of flavonoïds and phenolic compounds (R2 = 0.9412, p = 0.05).
Indeed, the phenolic content of a plant depends on a certain number of intrinsic factors (genetic) and extrinsic (climatic conditions, cultural practices, maturity with havest and conditions of storage) [14] (Table 1).
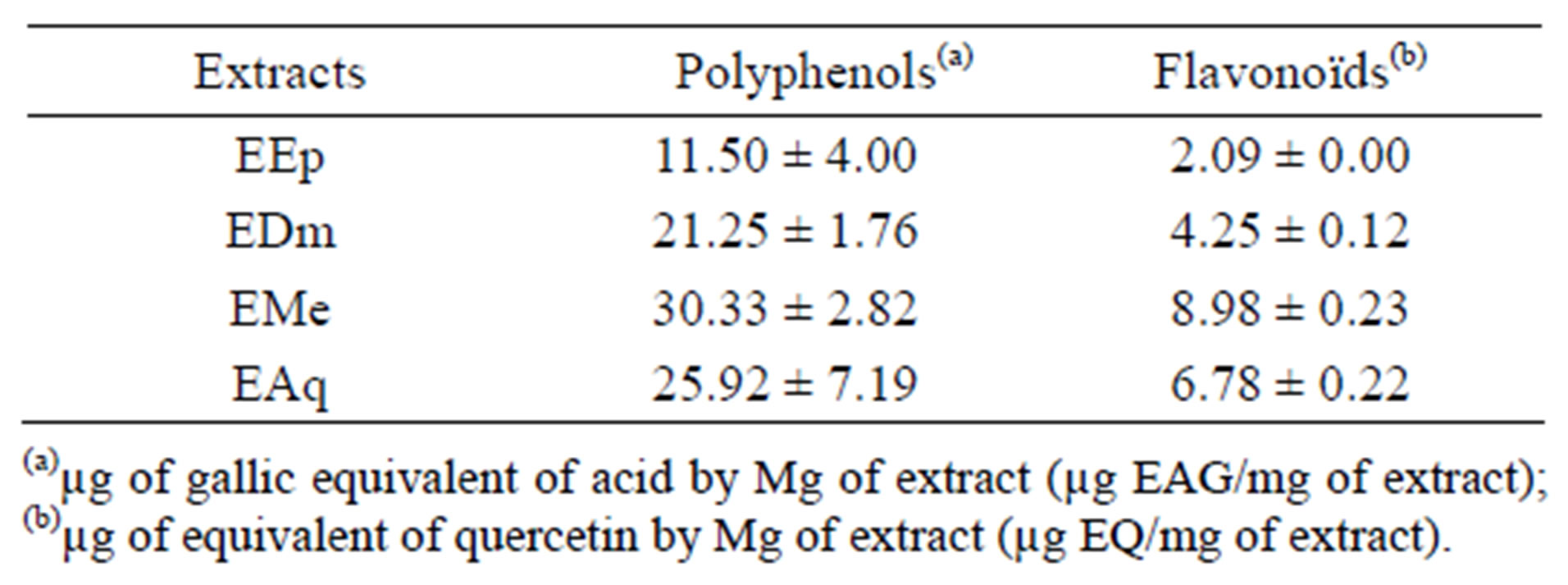
Table 1. Results of total phenolic content and the total flavonoïds in the extracts of Hertia cheirifolia.
3.2. Antioxidant Activity
3.2.1. Antioxidant Activity by Beta-Carotene Bleaching Test
The extract methanolic (EMe) shows the inhibiting activity highest whose its relative activity significantly remains lower (p ≤ 0.001) than that of the tocopherol (100%) used like controls positive.
An intermediate antioxydant activity was obtained with the aqueous extract (EAq) (67.56%) followed by extract dichlomethanolic (54.05%) which is statistically higher (p ≤ 0.001) than that of the extract etheric (33.78%) which represents the least active extract (Figures 1 and 2).
The polar extracts are especially rich in water-soluble chemical substances, their antioxydant activity shown by this method (BBC), which can be happened especially to the presence of the flavonoïds in these extracts.
This bond remains conditioned by the structure of the flavonoïds, particularly substitution hydroxy for the aromatic rings A and B and the model of substitution of the ring C, the most active flavonoïds have from 3 to 6 groups of hydroxyls [15]. On the other hand the glycolsylation of the flavonoïds reduces their capacities to trap the free radicals. As an example the antioxydant capacity increases kaempferol, quercetin with the myricétine, which coincides with the increase in the model of hydroxylation [16].
Moreover, Ferreira et al (2006) noticed that the inhibition test of linoleic acid oxydation coupled to that of the β-carotene, appears very useful like a mimetic model of lipid peroxidation in biological membranes.
3.2.2. Antioxidant Activity by DPPH Method
The DPPH free radical method is easy, fast and significant to examine the antioxydant activity of a specific compound or extracts [17].
The chemical compound 2,2-diphenyl-1-picrylhydrazyle (α,α-diphenyl-β-picrylhydrazylе).
Our results expressed as a percentage of the anti-ridicalizing activity (Figure 3), reveal that all the extracts tested as well as the standards are the ridicalizing ones.
Quercetin was taken as reference because it has the anti-ridicalizing activity highest (92.10%) compared to the other standards except the gallic acid which has a

Figure 1. Change of absorptance of the β-carotene to 490 nm in the presence of the extracts of Hertia cheirifolia tocopherol and negative control. Values are means ± SD of three replicates.
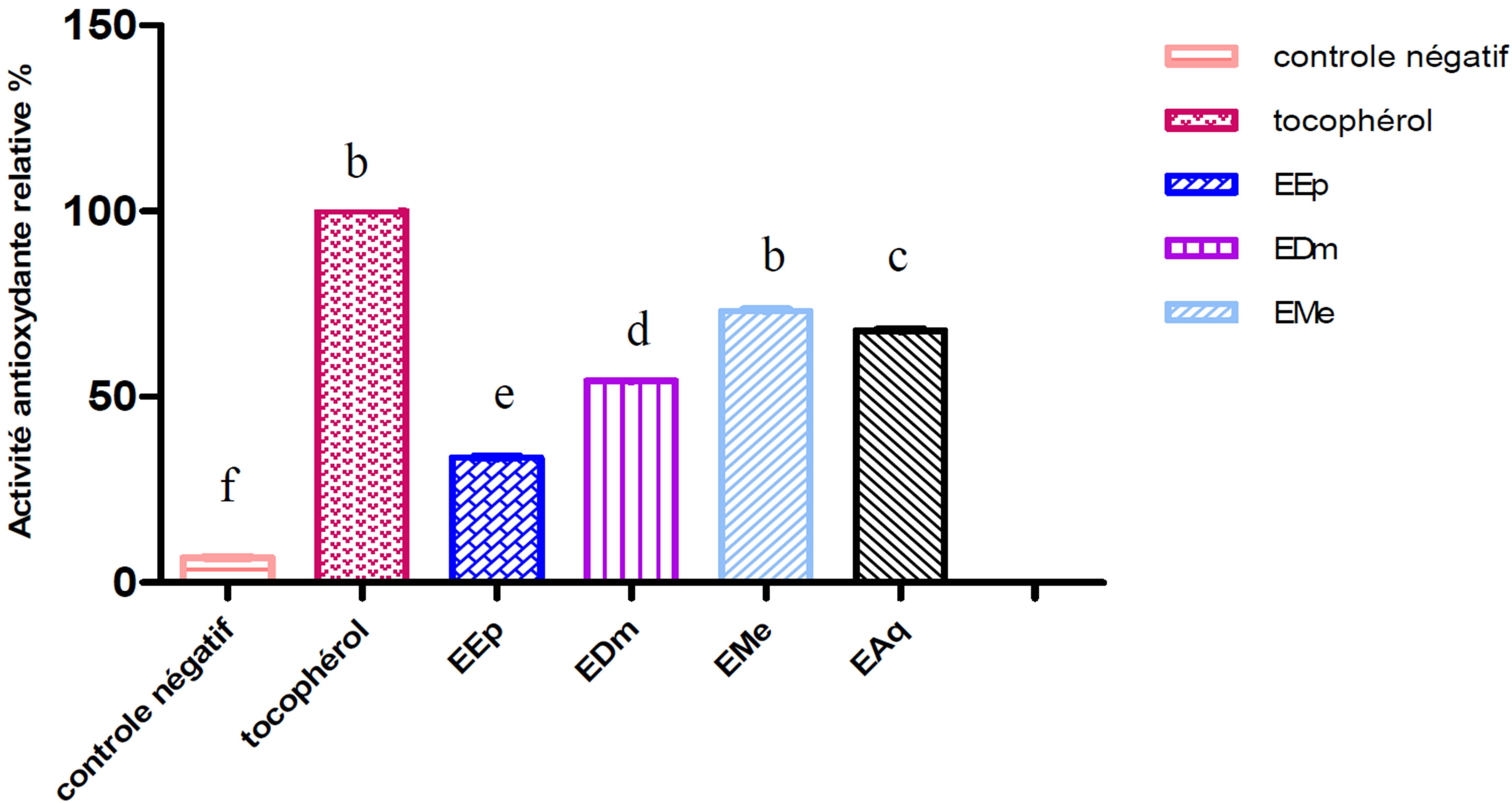
Figure 2. Relative antioxydant activity of the extracts of Hertia cheirifolia tocopherol and negative control. Values are means ± SD of three replicates.
The bars with different letters indicate activities significantly different (p < 0.05).

Figure 3. Anti-ridicalizing activity of the extracts of Hertia cheirifolia and the standards. Values are means ± SD of three replicat.
The bars with different letters indicate activities significantly different (p ≤ 0.05).
anti-ridicalizing activity (90.95%) almost identical to that of quercetin.
The EMe extract presented the highest anti-ridicalizing activity (72.74%), followed by EAq (63.64%), EDm (52.38%) and in the last EEp (47.14%).
These results almost are confused with those obtained in the case of BBC.
It was also noted that EMe and EAq, presented a raised antioxydant activity. This reducing activity can be explained by the presence of high rate of polyphenols [18, 19].
A linear correlation between the polyphenols content of Hertia cheirifolia’s extracts (R2 = 0.9160, p ≤ 0.05) and their anti-ridicalizing activity.
A linear correlation between the flavonoids content of the Hertia cheirifolia’s extracts (R2 = 0.8082, p ≤ 0.05) and their anti-ridicalizing activity.
The anti-radicalar activity of phenolic compounds depend on their molecular structure and the availability of phenolic hydrogen [20].
Many studies established relations between the chemical structures of the flavonoids and their antioxydants capacities, the activity of these molecules to trap the free radicals depends primarily on their structures and in particular the hydroxyl position, the molecule can act like a proton giving and show a radical sweeping activity [21, 22].
The most active flavonoids are those which contain groupings 3’ - 4’ dihydroxy on the cycle B and/or a grouping 3 OH on the cycle C [23].
Antibacterial activity
According to the results obtained, we prove that there is no antibacterial activity with the differents concentrations (Table 2).
Like was brought back in the literature, we considered that an extract has a bacteriostatic action if its diameter of inhibition is higher than 12 mm [24].
It noted that the extracts (EEp and EMe) shown a zones of inhibition on the bacterium Pseudomonas aeruginosa which are respectively (0.5 ± 0.66, 0.2 ± 0.54) but these zones of inhibition are induced by the solvent DMSO which has itself shown a zones of inhibition in control.
These results obtained can be allotted to the other factors such as the methods of extraction [25], preparation of the extract, used solvent, the stock tested, and finally the body of the plant used [26,27].
Thus [28,29] showed that the method used for the evaluation of the antibacterial activity influences the results These authors noted that the method of diffusion starting from the wells of gelose is advantaged to study the activity of the aqueous and organic extracts than the method of diffusion in gelose directely.
This test is fast and simple to carry out, however, it
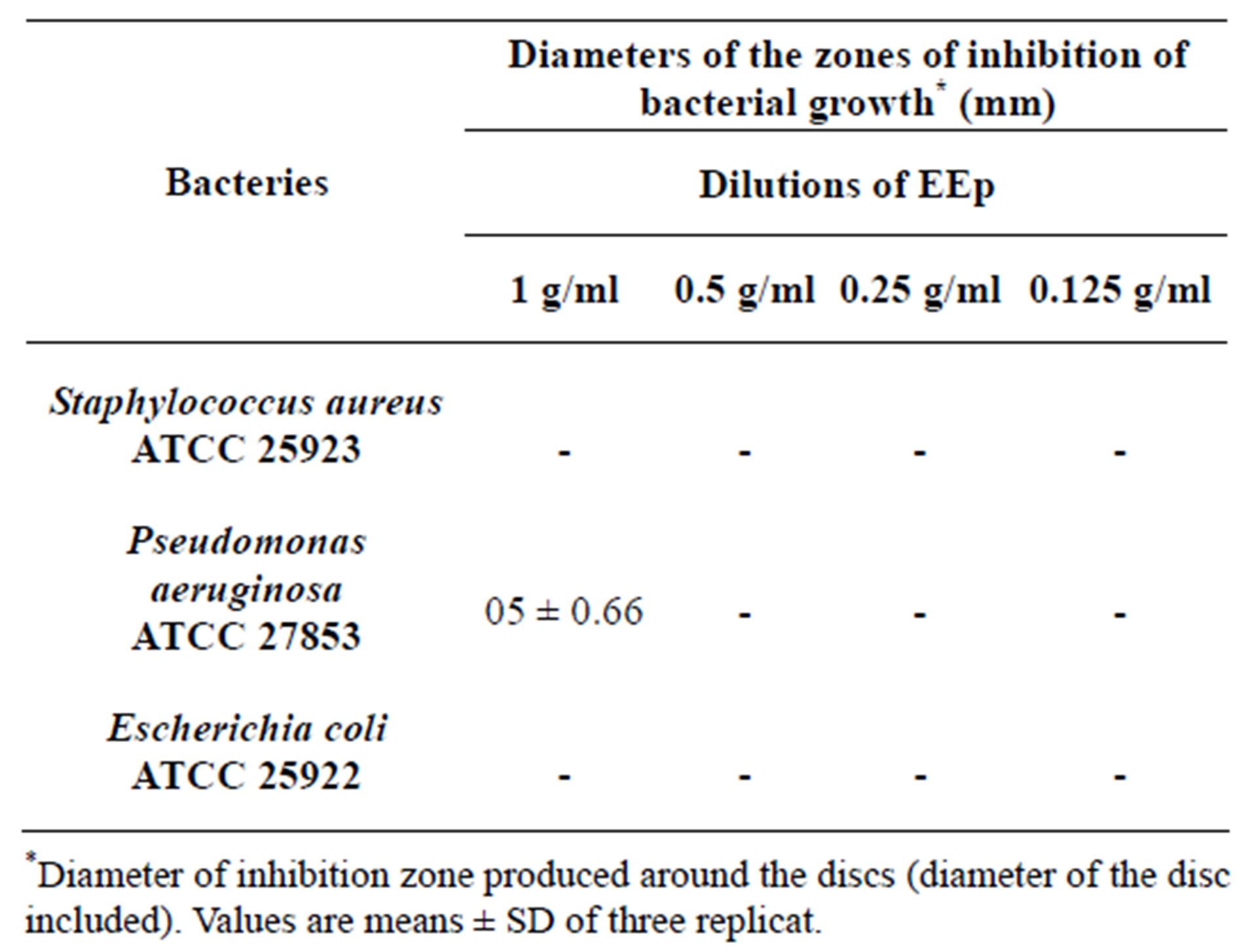
Table 2. Antibacterial activity (zone of inhibition) of EEp extract of the Leaves from Hertia cheirifolia.
would be judicious to set up tests of antibacterial with the method of dilution in order to have more rigorous results and more objectives.
4. CONCLUSIONS
The results of discolouration test of β-carotene showed an Inhibition activity of the coupled linoleic acid oxidation β-carotene for the whole extracts of Hertia cheirifolia, this activity significantly remains lower than the positive control. The results of the test with the DPPH revealed that the extracts of Hertia cheirifolia present very significant anti-ridicalizing activities; however, the activity of MeOH is higher than the quercetin. The results obtained are related directly to quantitative diversity and/or qualitative of the compounds present in the extracts of Hertia cheirifolia. This study contributes to the knowledge of the potentials antioxydant and antibacterial in vitro of Hertia cheirifolia.
• Insulation and characterization of the active compounds in the various extracts by more specific methods like the NMR.
• For better evaluating the antioxydant activity, other in vitro studies and in vivo would be interesting.
• The test on other bacterial and fungic stocks.
• Evaluation of the anti-inflammatory drug activity and hepatop rotective in vivo.