1. Introduction
Soil conditions are important determinants of biodiversity both above and below the ground level and influence ecology and angiosperm evolution. Extreme soils tend to evolve organisms that are specifically adapted to the offered abiotic conditions [1]. Serpentine soils, which are derived from ultramafic rocks, are typically low in essential macronutrients (nitrogen, potassium, and phosphorous) and exhibit toxic concentrations of heavy metals such as nickel as well as unbalanced calcium-to-magnesium ratios [2]. These hostile conditions impose strong limits on species establishment and diversity, most notably among plants. Serpentine flora are characterised by low productivity, species richness, high endemism, and ecotypic specialisation [3]. Plants that are specialised in the serpentine environment tend to be poor competitors on other specialised substrates [3]. Although they perform better on non-serpentine soils, serpentine specialists experience a trade-off regarding competitive ability, which leads to exclusion from non-serpentine habitats by their native species [1].
Some species adapt to the serpentine environments of Japan. Among them, Aster hispidus Thunb. var. leptocladus (Makino) Okuyama (Asteraceae) is one of the species endemic to the serpentine soils and is distributed in Shikoku, Kyushu, and Honshu (Figure 1) [4,5]. This species is distinguished from its closely related taxa, A. hispidus var. hispidus, A. hispidus var. insularis (Makino) Okuyama and A. hispidus var. koizumianum (Kitam.)
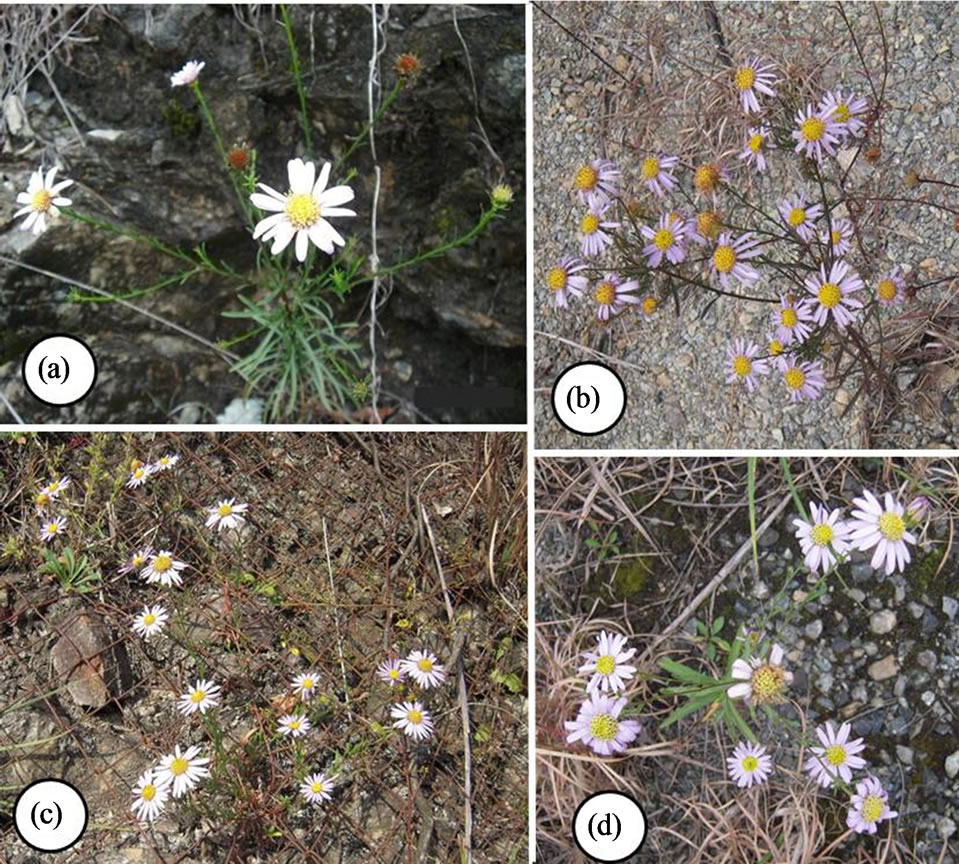
Figure 1. Aster hispidus var. leptocladus: (a) Ikku; (b) Shingu; (c) Hidaka; and (d) Aichi.
Okuyama, by narrower leaves, thinner stems, and shorter puppus [4]. A recent molecular analysis using nuclear microsatellite sequences revealed that A. hispidus var. leptocladus in Shikoku, Kyushu, and Honshu were involved in different lineages [5], indicating that these groups had originated independently in these areas. As for the parallel evolution of narrow leaves in A. hispidus var. leptocladus, it is not known whether this species in different lineages had the same anatomical processes, since no such studies have been conducted. Therefore, to clarify the question of anatomical leaf differentiation, we characterised leaf cell variation in morphological and anatomical analyses.
2. Materials and Methods
Samples of Aster hispidus var. hispidus and A. hispidus var. leptocladus examined in this study were collected from the field (Figure 2; Table 1). Voucher specimens were deposited in the herbarium of the Makino Botanical Garden, Kochi (MBK).
For morphological analysis, we measured the lengths and widths of individual leaf blades. We analysed 226 leaves from 15 A. hispidus var. hispidus samples and 1233 leaves from 121 A. hispidus var. leptocladus samples (Ikku: 320 leaves from 17 individuals; Shingu: 175 leaves from 22 individuals; Hidaka: 243 leaves form 27 individuals; and Aichi: 495 leaves from 55 individuals). Measurements were conducted using a digital caliper. Fully expanded stem leaves were used for leaf measurements.
For anatomical analysis, we calculated leaf index, which is the ratio of length to width, from the initial measurements to clarify leaf shape. To count the number of cells on the blade, the surface of each leaf was replicated using Suzuki’s Universal Micro-Printing (SUMP) method. The middle part of the blade along the midrib was analysed to determine the number of cells on the blade. The same SUMP image was analysed 10 times for each trait using a light microscope.
Statistical analysis was performed using Tukey’s honestly significant difference (HSD) test to compare the measurements. Because the leaf index in either population was not normally distributed, nonparametric pairwise comparison was conducted (Steel-Dwass test) [6,7].
3. Results
3.1. Morphological Measurements of Aster hispidus var. leptocladus and A. hispidus var. hispidus
A. hispidus var. leptocladus generally had shorter leaf length than A. hispidus var. hispidus (Table 2). All localities of A. hispidus var. leptocladus had significantly narrower leaf widths than those of A. hispidus var. hispidus (4.69 ± 1.35 mm). A. hispidus var. leptocladus in Ikku (2.20 ± 1.12 mm), Shingu (1.55 ± 0.42 mm), and Aichi (2.06 ± 0.40 mm) had the narrowest leaves in this study. Thus, the order of leaf width was A. hispidus var. hispidus > Hidaka > Ikku = Aichi = Shingu. We also calculated the leaf index value as specified by Tsukaya [8]. Although the leaf indices of Ikku (10.5 ± 3.5) and Shingu (10.3 ± 2.2) of A. hispidus var. leptocladus were higher than that of A. hispidus var. hispidus (6.9 ± 1.8), those of Hidaka (6.8 ± 1.3) and Aichi (6.9 ± 1.6) were the same as that of A. hispidus var. hispidus.
3.2. Epidermal Cells of A. hispidus var. leptocladus and A. hispidus var. hispidus
The epidermal cell length in A. hispidus var. leptocladus was significantly longer than that of A. hispidus var. hispidus (38.86 ± 6.61 µm) (Table 2). A. hispidus var. leptocladus in Shingu and Hidaka had the longest epidermal
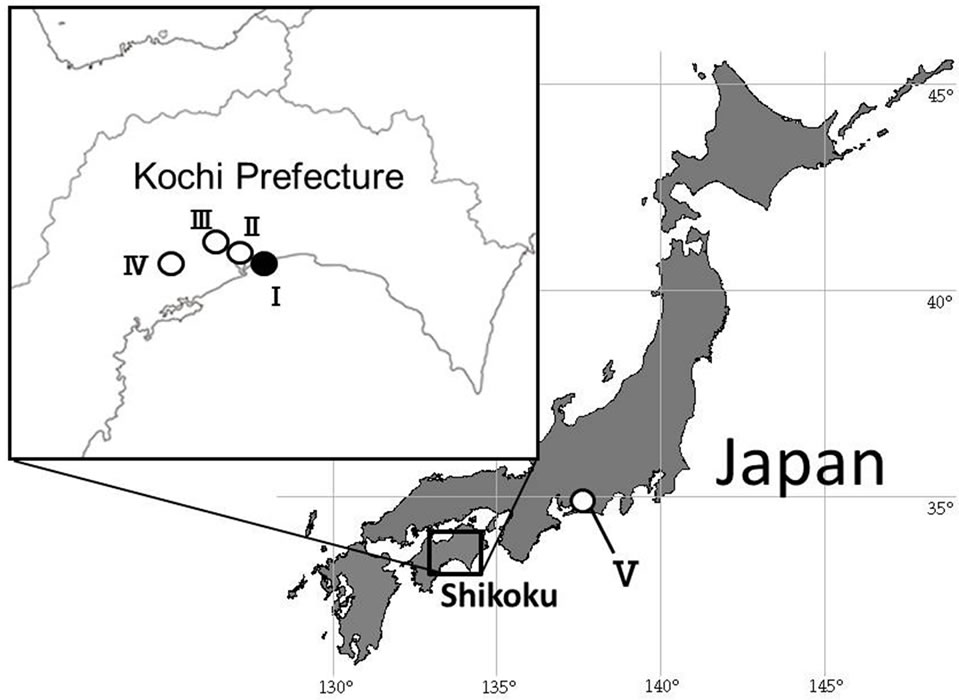
Figure 2. Sampling localities used in this study. See Table 1 for other abbreviations.

Table 1. Sampling localities used in this study.
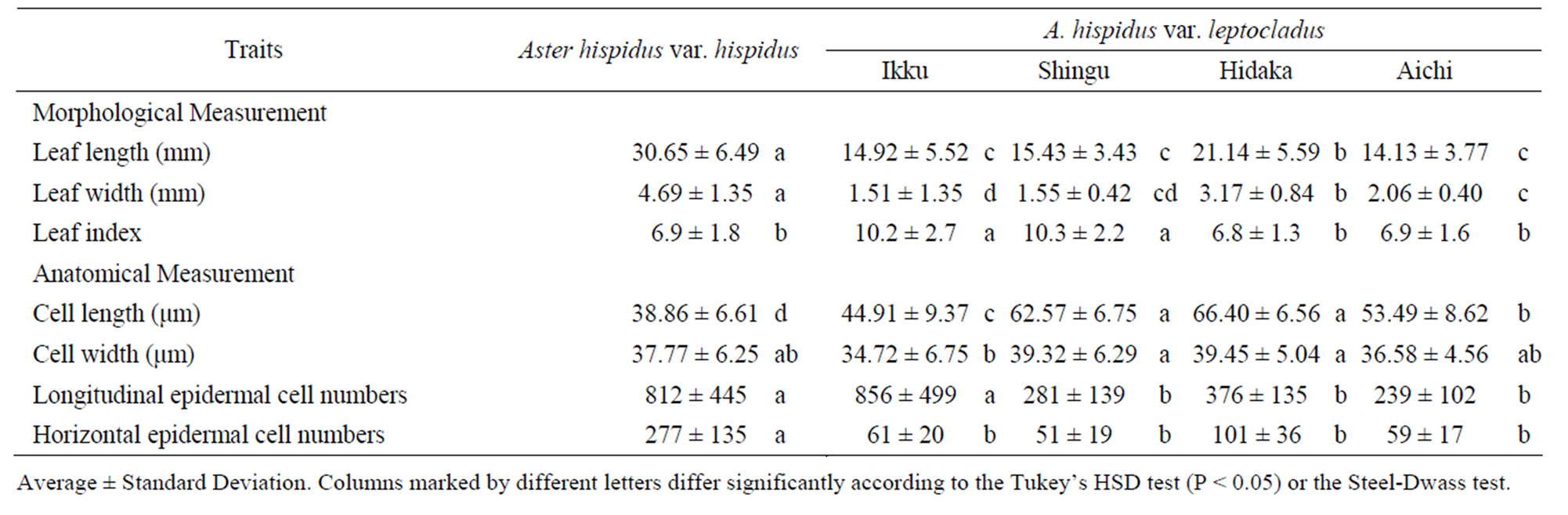
Table 2. Result of the leaf measurements.
cell sizes (62.57 ± 6.75 µm and 66.40 ± 6.56 µm, respectively) in this study. The epidermal cell width of A. hispidus var. leptocladus did not differ significantly from that of A. hispidus var. hispidus. The longitudinal epidermal cell number estimated by dividing leaf width by epidermal cell size was 856 ± 499 in Ikku, 281 ± 139 in Shingu, 376 ± 135 in Hidaka, and 239 ± 102 in Aichi for A. hispidus var. leptocladus and 812 ± 445 for A. hispidus var. hispidus. Although A. hispidus var. leptocladus in Ikku had same number of epidermal cells in the longitudinal direction as A. hispidus var. hispidus, A. hispidus var. leptocladus in Shingu, Hidaka, and Aichi had fewer cells than did A. hispidus var. hispidus. The horizontal epidermal cell number was 61 ± 20 in Ikku, 51 ± 19 in Shingu, 101 ± 36 in Hidaka, and 59 ± 17 in Aichi for A. hispidus var. leptocladus and 277 ± 135 for A. hispidus var. hispidus. All localities of A. hispidus var. leptocladus had significantly fewer epidermal cells in the horizontal direction than A. hispidus var. hispidus.
4. Discussion
Our morphological results indicated that both width and length of Aster hispidus var. leptocladus from all the areas that we studied were significantly smaller than those of A. hispidus var. hispidus. However, the leaf indices of individuals from the Ikku and Shingu populations were significantly higher than those of the others. These results revealed that the leaves of A. hispidus var. leptocladus from the Aichi and Hidaka populations were more minitualized but those from Ikku and Shingu were more stenophyllized than the leaves of A. hispidus var. hispidus. Of these, leaf stenophyllization is generally seen in rheophytic species, i.e., in those that inhabit areas along the rivers, and has occurred independently in various plant families [9] to avoid selection pressure from flooding rivers [10]. Therefore, it is quite interesting that similar morphological leaf modifications occurred in those closely related taxa under different selection pressures of the serpentine soil and flooding frequency of river-stream.
Our anatomical results indicated that the decreased number of cells in the horizontal axis could be attributed to the linear leaves in A. hispidus var. leptocladus. However, individuals from the Shingu, Hidaka, and Aichi populations had more decreased cell numbers in the longitudinal axis and more increased cell lengths compared to those of Ikku. As for the anatomical process that forms stenophyllization, Usukura et al. [11] reported that the narrow leaves of the rheophyte Farfugium japonicum (L. fil.) Kitam. var. luchuense (Masam.) Kitam. (Asteraceae) might have evolved due to a decreased number of leaf cells across the leaf width. Moreover, Setoguchi and Kajimura [12] indicated that speciation from Rhododendron indicum (L.) Sweet f. indicum to rheophytic R. indicum f. otakumi T. Yamaz. (Ericaceae) was involved in the decreased number of leaf cells. Therefore, we believe that the stenophyllization of A. hispidus var. leptocladus in the Ikku and Shingu populations had a similar anatomical process to those noted in the studies using the rheophyte species. In addition, we found that minitualization of the leaves of A. hispidus var. leptocladus in the Aichi and Hidaka populations had a modification pattern other than stenophyllization, the unique anatomical process used to adapt to serpentine soils.
Recently, Yokoo et al. [5] indicated that the Aichi and Kochi populations of A. hispidus var. leptocladus were composed of paraphyletic assemblages and that the Kochi populations of this species had a single origin. In our study, different processes form the narrow leaves of A. hispidus var. leptocladus in Kochi, and individuals of the Aichi and Hidaka populations have similar processes to do so. Therefore, we could not clarify whether Aichi and Kochi of A. hispidus var. leptocladus originated independently and yet had similar anatomical process; this species possibly developed a different mechanism to form the narrow leaves in Kochi.
In summary, we analysed A. hispidus var. leptocladus using morphological and anatomical data. These results contribute to an unbiased interpretation of the morphological adaptation to serpentine soils. Additional examination of samples such as those of the Kyushu population will further illuminate the mechanisms by which A. hispidus var. leptocladus differentiates itself from A. hispidus var. hispidus.
5. Acknowledgements
We wish to thank Muramatsu Y, Hirata A, Ohga K, Yoshimi Y, Muroi M, Yokoyama N, Matsuyama K, Isomoto S, Miyata H, Tsuchiya Y, Kumekawa Y and Hamachi H for providing much help. This study was partly supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science and Culture of Japan (to TF, JY and RA).
NOTES