Light Scattering and SAXS Study of AOT Microemulsion at Low Size Droplet ()
1. Introduction
Brownian motion is a subject of renewed interest since the development of photon correlation spectroscopy (PCS) in the last decade. The dynamic properties of microemulsions and colloidal systems is studied by measuring the relaxation of concentration fluctuations, [1,2]. The relaxation time of the fluctuations can be expressed in a diffusion coefficient, which is called the collective diffusion coefficient (Dc), in the limit of infinite dilution. The study of the collective diffusion coefficient of microemulsion is an interesting topic in soft matter physics. The microemul- sions are thermodynamically stable mixtures of water, oil, and surfactant with nano-metric size of droplets to the solvents (H2O or Oil). In the case of water/surfactants (droplet) to the nanopolar solvents (oil), microemulsions have L2 phase and oil/surfactant inside the water solutions is L1 phase of microemulsions. In our samples, the composition of each system is determined by the molar ratio X of water to surfactant molecules.
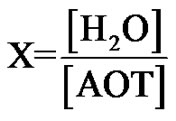 (1)
(1)
and the droplet mass fraction (mf),
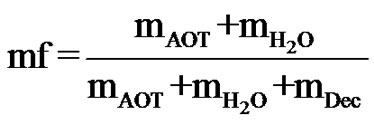 (2)
(2)
which varies by the respective mass of the components water ( ), decane (mDec), and AOT. In this work, the microemulsion system is located in the L2 region at studied temperatures and compositions investigated. Most works on collective diffusion coefficient of droplets and interactions of droplets have been done mainly on charged macromolecules, [3-15], for example bimolecular, [3-10], as DNA, proteins and amino acids, micelles, [11], and polymer latexes, [15], in water (as polar solvent) at low ionic strength. The strong, long range electrostatic interactions between the particles or droplets in these systems lead to remarkable structure effects. Only in the higher number densities or droplet concentration of microemulsions where the inter-particle separation is in the order of the particle dimensions hydrodynamic interactions can be observed.
), decane (mDec), and AOT. In this work, the microemulsion system is located in the L2 region at studied temperatures and compositions investigated. Most works on collective diffusion coefficient of droplets and interactions of droplets have been done mainly on charged macromolecules, [3-15], for example bimolecular, [3-10], as DNA, proteins and amino acids, micelles, [11], and polymer latexes, [15], in water (as polar solvent) at low ionic strength. The strong, long range electrostatic interactions between the particles or droplets in these systems lead to remarkable structure effects. Only in the higher number densities or droplet concentration of microemulsions where the inter-particle separation is in the order of the particle dimensions hydrodynamic interactions can be observed.
In previous study of the collective diffusion coefficient in C12E5/H2O/Decane microemulsion, the collective diffusion coefficient as function of droplet mass fraction has positive slop at the low droplet mass fraction (dilute regime mass fraction below 0.1) that describes an attractive interaction between droplets. It is well known that C12E5/- H2O/Decane microemulsion at constant surfactant-oil mass ratio of 1.08 with the different mass fractions has shown behaviors as the hard sphere droplets, [16-20]. The Collec- tive diffusion coefficient as function of mass fraction in the C12E5/H2O/Decane microemulsion has positive behavior and only depends to the inter-particle interaction of hard sphere droplet. The mixture of PEG with C12E5/H2O/- Decane microemulsion shown that addition of PEG causes slow down of the collective diffusion coefficient Dc. The measured Dc data for the droplets in the presence of PEG are modeled using the Asakura-Oosawa theory of depletion, [20], but Dc in the AOT/H2O/Decane microemulsions has different behavior. Structure and phase behavior of AOT microemulsions are well investigated of a liquid of droplets on a nanometer scale, [21]. A study shown, for AOT/H2O/Decane microemulsion, at the low droplet mass fractions (0.01 < mf < 0.1 transition from dilute to semi-dilute regime) and molar ratio 40 the collective diffusion coefficient has a linear function of mass fraction. It is well known that at high water concentration with the molar ratio of X = 40 the collective diffusion coefficient Dc depends on the droplet mass fraction (droplet concentration) due to inter-particle interactions, [22]. This study is an attempt to understand the collective diffusion coefficient behavior of AOT/H2O/Decane microemulsion at low water concentration (molar ratio, X = 6.7). The most study in this range of water concentration that shown droplet of the microemulsion has spherical behavior, [23,24]. In the present work, we studied the behavior of the collective diffusion coefficient at the molar ratio 6.7 and low mass fraction (0.01 < mf < 0.08), by means of DLS and SAXS techniques. Also, we study effect of TBAC on the Dc in the AOT/H2O/Decane microemulsion.
2. Experimental
2.1. Methods and Materials
Sodium-2-diethylhexyl sulfosuccinate, or AOT 99% (an Alfa product), was dried in vacuum. Deionized and distilled water were employed to prepare the samples for the light scattering and SAXS measurements and solutions of the mix TBAC with microemulsions. The microemulsions were prepared by mixing of surfactants AOT, H2O, and oil (Decane) and waiting for several minutes until the samples were single phase and optically clear. Decane 99% (Aldrich) and tetrabutylammonium chloride (TBAC) and n-decane were obtained from Sigma-Aldrich. The composition of the AOT/H2O/Decane microemulsion is given by the two parameters X and mf. The mixing of TBAC with microemulsions is described with the molar ratio of TBAC to AOT, Equation (3).
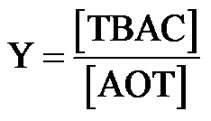 (3)
(3)
The experimental solutions were prepared at temperature 293.15 K. The microemulsions were prepared by weighting appropriate amounts of AOT dissolving in decane then by adding water into the solution. Finally, all samples were filtered by using 0.2-pm Teflon filters (Gelman). The Small-angle X-ray scattering measurements were performed using the pinhole SAXS instrument at the University of Aarhus. The instrument consists of an X-ray camera (NanoSTAR, Bruker AXS) with a rotating anode X-ray (Cu Kα radiation) source, cross-coupled Göbel mirrors, collimation using three pinholes and an evacuated beam path, and a 2D position-sensitive gas detector (HiSTAR). In the current experiments the small pinholes were used, giving a range of scattering vectors as 0.0084 ≤ Q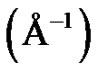 ≤ 0.35, where Q =
≤ 0.35, where Q = 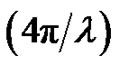 sin
sin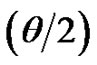 is the modulus of the wave vector, θ is the scattering angle, and λ = 1.542 Å is the X-ray wavelength. The sample was held in a 1 mm glass capillary at room temperature. Data was acquired as a function of the scattering vector modulus
is the modulus of the wave vector, θ is the scattering angle, and λ = 1.542 Å is the X-ray wavelength. The sample was held in a 1 mm glass capillary at room temperature. Data was acquired as a function of the scattering vector modulus 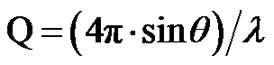 where 2θ is the angle between the incident and scattered photons. Dynamic light scattering measurements were performed on filtered samples using ALV CGS-8f/DLS Instruments Series A6160-V1052 at Ulm University. The scattering angle was maintained at 90˚, and the temperature was kept at 293.15 K for the microemulsion samples.
where 2θ is the angle between the incident and scattered photons. Dynamic light scattering measurements were performed on filtered samples using ALV CGS-8f/DLS Instruments Series A6160-V1052 at Ulm University. The scattering angle was maintained at 90˚, and the temperature was kept at 293.15 K for the microemulsion samples.
3. Results
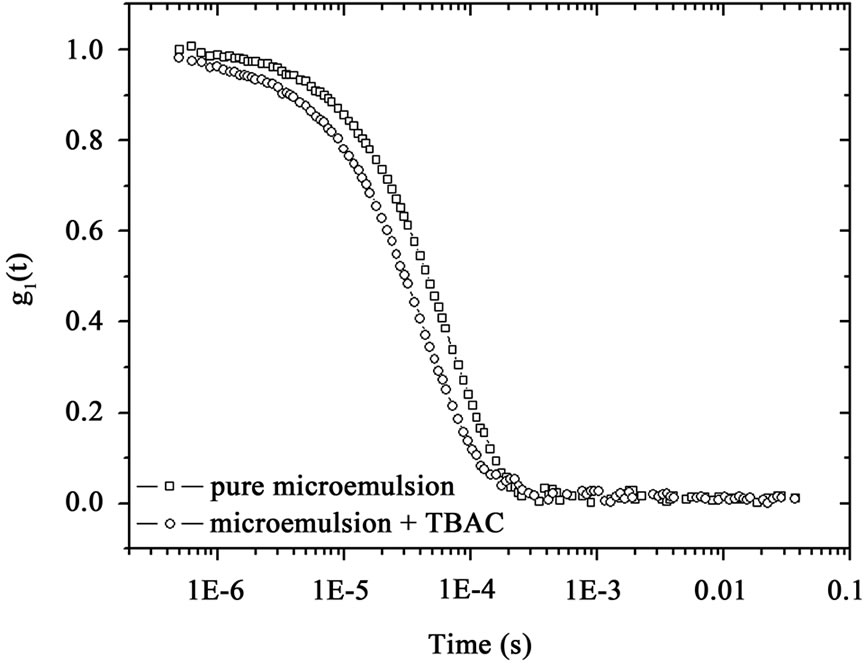
Microemulsions were formulated by mixing AOT with water and n-Decane at the fixed molar ratio of water to AOT (X = 6.7) at the different mass fraction (0.01 < mf < 0.07 dilute regime). The dynamic behavior of the AOT/- H2O/Decane microemulsion was probed with dynamic light spectroscopy. The correlation function of the scattered light intensity showed a single exponential decay at all concentrations, Figure 1. (A). All the correlation functions in this work were fitted by a single stretched exponential function, [1,2].
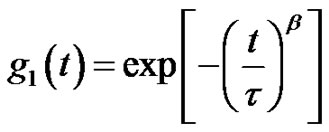 (4)
(4)
That 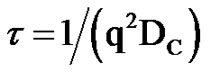 and the collective diffusion coeffi- cient Dc was extracted as a function of the droplet mass fraction as shown in Figure 1(b). The collective diffusion coefficient measurements were carried out on dilution series of microemulsion samples at fixed temperature 293.15 K and molar ratio 6.7 with two different molar ratio of TBAC (0.0 and 0.049 molar/l). In the Figure 1, the collective diffusion coefficient show a liner behavior with negative slop between 0.01 < mf < 0.07. By increasing TBAC content relaxation time decreases, Figure 1(a) and the diffusion coefficient increases, Figure 1(b).
and the collective diffusion coeffi- cient Dc was extracted as a function of the droplet mass fraction as shown in Figure 1(b). The collective diffusion coefficient measurements were carried out on dilution series of microemulsion samples at fixed temperature 293.15 K and molar ratio 6.7 with two different molar ratio of TBAC (0.0 and 0.049 molar/l). In the Figure 1, the collective diffusion coefficient show a liner behavior with negative slop between 0.01 < mf < 0.07. By increasing TBAC content relaxation time decreases, Figure 1(a) and the diffusion coefficient increases, Figure 1(b).
In this experiment, SAXS is used for the determination of the structure of AOT/H2O/Decane microemulsions at 293.15 K. The SAXS methods are useful for studies the structure of reverse micelles. This is because the scattering of X-rays of wavelengths of a few angstroms through small angles provides a Q range (as defined by Equation 2) which is particularly appropriate for the determination of both
 (a)
(a)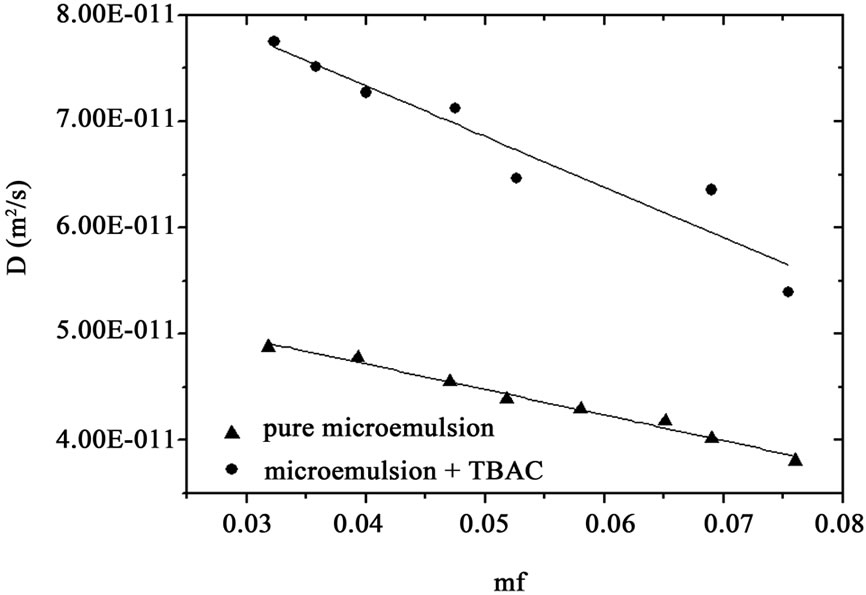 (b)
(b)
Figure 1. (a) Autocorrelation function before and after mixture of TBAC with AOT/H2O/Decane microemulsion (b) The collective diffusion coefficient as a function of mass fraction (mf) for water to AOT and Decane with X = 6.7 (circle) and X = 6.7, [TBAC]/[AOT] = 0.049 molar/l (Down triangle) at the temperature 293.15 K.
the size of discrete reverse micelles and their interactions. From the angular dependence of the scattered X-ray intensity 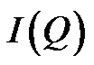 we have
we have
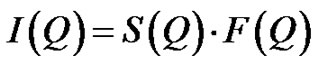 (5)
(5)
where
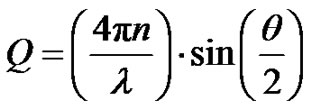 (6)
(6)
Q is the wavelength and θ is the scattering angle. 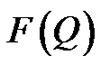 is referred to the form factor, which gives information on the dimensions of an individual reverse micelle, and
is referred to the form factor, which gives information on the dimensions of an individual reverse micelle, and 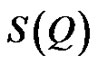 is the structure factor, which provides information on inter-micelle interaction. We will consider the nature of the interactions between micelles. Information on interactions can be obtained from structure factor. The scattered intensity as a function of Q from samples presented in the Figure 2, which the lines are fits to a power law,
is the structure factor, which provides information on inter-micelle interaction. We will consider the nature of the interactions between micelles. Information on interactions can be obtained from structure factor. The scattered intensity as a function of Q from samples presented in the Figure 2, which the lines are fits to a power law,
 (a)
(a)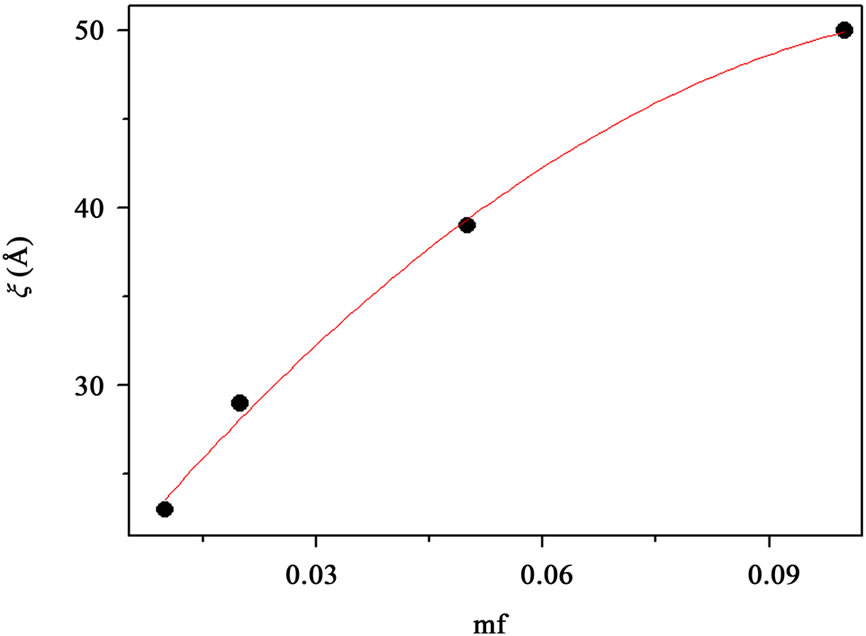 (b)
(b)
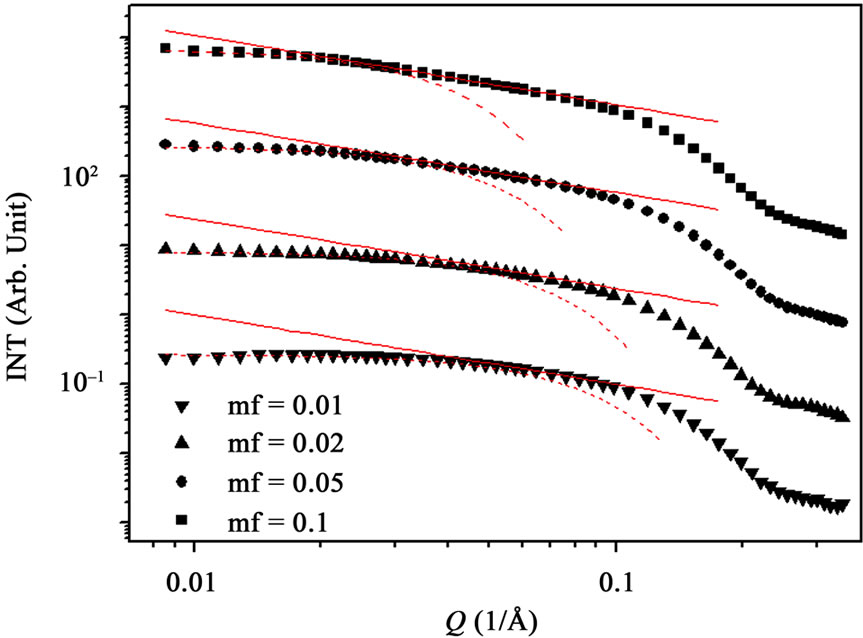
Figure 2. (a) SAXS intensity I(Q) of a AOT/H2O/Decane microemulsion with X = 6.7 and different droplet mass fraction (mf = 0.01, 0.2, 0.05, 0.1). The red line is the I(Q) ≈ Q–1 that shown cylinder behavior of the SAXS experiments and dot line at low Q is the fit of Guinier’s law, I(Q) ≈ exp (–(Qξ) 2/3). (b) The correlation length ξ (Å) as a function of droplet mass fraction for AOT/H2O/Decane microemulsion with X = 6.7. The red line is fit with polynomial function.
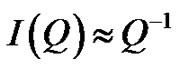 , with properties of a cylindrical object. At small Q, the scattering is only sensitive to the overall dimension of the scattering particles, and we expect from Guinier’s law,
, with properties of a cylindrical object. At small Q, the scattering is only sensitive to the overall dimension of the scattering particles, and we expect from Guinier’s law, 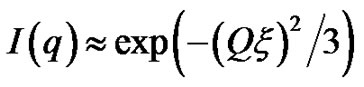 , where ξ is correlation length. The microemulsions with molar ration 6.7 and different droplet mass fractions were collected for these experiments. The SAXS data is measured from the high concentration sample (mass fraction 0.1) to the dilute sample. Figure 2 shows the small angle X-ray scattering intensities for AOT/H2O/Decane as a function of Q at the molar ratio 6.7 and different mass fraction.
, where ξ is correlation length. The microemulsions with molar ration 6.7 and different droplet mass fractions were collected for these experiments. The SAXS data is measured from the high concentration sample (mass fraction 0.1) to the dilute sample. Figure 2 shows the small angle X-ray scattering intensities for AOT/H2O/Decane as a function of Q at the molar ratio 6.7 and different mass fraction.
Figure 2, depict that intensity as a function of Q at the high droplet mass fraction (mf = 0.1) and Q range of 0.02 < Q (1/Å) < 0.1 has linear behavior. At low Q, we used Guinier’s law, 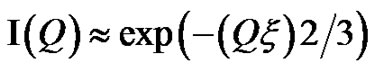 , that results presented in the Figure 3" target="_self"> Figure 3. Our results show, the correlation length
, that results presented in the Figure 3" target="_self"> Figure 3. Our results show, the correlation length 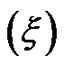 change from 23 Å to 50 Å with the increase of droplet mass fraction (Figure 3). The most important observation from Figure 2 is increasing of the correlation length with the increase of droplet mass fraction. We also study the effect of adding TBAC to the AOT/H2O/Decane microemulsion with SAXS experiment. The correlation length change from 50 Å to 30 Å with adds TBAC to the AOT/H2O/Decane microemulsion, Figure 3.
change from 23 Å to 50 Å with the increase of droplet mass fraction (Figure 3). The most important observation from Figure 2 is increasing of the correlation length with the increase of droplet mass fraction. We also study the effect of adding TBAC to the AOT/H2O/Decane microemulsion with SAXS experiment. The correlation length change from 50 Å to 30 Å with adds TBAC to the AOT/H2O/Decane microemulsion, Figure 3.