Drinking Water Treatment: pH Adjustment Using Natural Physical Field ()
1. Introduction
Water is known as a key component of the life of any living organism, but this source of life can be a curse in some cases and drinking water becomes a danger to people’s health [1]. In details, Loss et al., 2017 explained a report published by the Soil and Tillage Research, a specialist in which they said that some people suffer from many health problems due to drinking water [2]. Their study report attributed these problems to the fact that many people do not realize the value of what is called the “pH” of the water they drink, as the pH determines the concentration of hydrogen ions in the water, which determines the “acidity” of the water, which can cause problems. Many if it is high or low, depending on the health condition. Researchers warn that a low pH turns water into a toxic solution, and it is also more attractive to heavy metals in the human body.
The pH is lower, may cause digestive problems for some people, if the person suffers from high acidity in the stomach. This explains the increase in the popularity of potable alkaline water in the recent period, which leads to the neutralization of acids in the digestive system, and makes the person feel more comfortable. Alkaline water helps the body become more alkaline, which treats many diseases including cancer [3] [4]. Also, drinking alkaline water leads to an equalization of the pH of the mouth, digestive system, and urine, so its continued intake leads to an equalization of the pH of the whole human body. In turn, a study indicated that the use of alkaline water with a degree of 8.8 can disrupt the work of an enzyme in the body, which plays a dangerous role in heartburn, and reduces its annoying symptoms. She explained that alkaline water, which has a pH between 8.5 and 10 degrees, is beneficial for people with irritable bowel syndrome. It is noteworthy that the human body has a “fine natural regulator” of acidity levels, which cause changes in the pH of the blood, such as that which solve serious problems in the organs and tissues [5].
2. Literature Review
The pH of water indicates whether it is acidic or alkaline. The pH scale ranges from 0 to 14, with 7 indicating neutral. The pH of drinking water should be between 6 and 8.5. If the water is acidic, this treatment process is used (low pH) [6]. When pumped into a water system, soda ash (sodium carbonate) and sodium hydroxide increase the pH of the water to near neutral. They do not cause hardness problems in treated water, unlike neutralizing filters [7]. Baking soda’s pH cannot be measured since it is a dry powder. Since pH is determined by the relative amounts of free hydrogen and hydroxyl ions in a water-based solution, an aqueous solution is needed.
Researchers attempted to evaluate the impact of physically activated carbon to remove natural organic matter in drinking water purification process. Korotta-Gamage and Sathasivan (2017) highlighted that physically activated carbon (BAC) removes low molecular weight in natural organic matter (NOM). They also concluded that treatment with BAC is slow, and the effluent can contain soluble microbial products and microbes [8]. On the other hand, a great deal of previous research has focused on removing some compounds from the water, especially groundwater, in order to raise the pH of the water, making it alkaline. Bora and Dutta (2019) set up a series of experiments using a new technic to remove of metals (Pb, Cd, Cu, Cr, Ni, and Co) from drinking water by oxidation-coagulation-absorption at optimized pH. In fact, the removal of certain metals, such as Cd, Pb, Ni, Cr, Cu, and Co, from groundwater using the oxidation-coagulation-adsorption at optimised pH (OCOP) process, which is an effective and low-cost method for simultaneous removal of As, Fe, and Mn. In their study the process has been found to remove the selected metals from an initial concentration of 2 mg/L in the following order: Cd (79.0 percent), Co (94.8 percent), Ni (94.4 percent), Cu (98.0 percent), Cr (98.3 percent), and Pb (98.3 percent) (99.5 percent). The heavy metals adsorb on the surface of coagulates according to atomic adsorption spectrophotometer, energy distributed X-ray spectrophotometer, and powder X-ray diffraction analyses [9].
Hashim et al., (2019) demonstrated that the use of an electrocoagulation (EC) reactor with aluminium electrodes is used to investigate the effect of the initial pH on the removal of reactive black 5 dye (RB5) from water. In their study several sets of continuous flow experiments were started at five different initial pH values (4, 5, 6, 7, and 8) while keeping the current density, inter-electrode size, and RB5 concentration constant at 2 mA/cm2, 4 mm, and 25 mg/L, respectively. The most obvious finding to emerge from this study is that the removal efficiency increased steadily as the initial pH increased from 4 to 6, reaching a maximum of 96% at the neutral pH range, before decreasing to 74% as the initial pH increased to 8 pH value [10].
Not only drinking water needs to raise pH levels, but also fish farming water needs to raise pH levels, and this is what we find in a study by Martins et al., (2017) as sodium bicarbonate (NaHCO3), calcium carbonate (CaCO3), and calcium hydroxide (Ca(OH)2) were used to test alkalinity and pH maintenance on BFT during a Nile tilapia (Oreochromis niloticus) nursery. The findings show that using sodium bicarbonate, hydroxide, or calcium carbonate to change alkalinity and pH in the final proportions of 14.64, 0.49, 7.18, 0.32, and 24.09, 2.32 percent in relation to feed consumption, respectively, is accurate [11].
It is now well established from a variety of studies it becomes clear to us beyond a reasonable doubt that there is no scientific study that has carried out the process of raising the pH in a physical field without chemical intervention. Therefore, this study tries to test the effect of the biosphere for heat treated silica under certain conditions in raising the pH of drinking water and helping to remove harmful bacteria in it.
3. Research Methodology
The amount of hydrogen ions present in water is measured by the pH of the water. It decides whether the water is acidic or alkaline. The term pH refers to the hydrogen potential. The pH of water should be between 6.5 and 8.5, according to the World Health Organization (WHO). pH can be determined using the following formula: pH = −log [H]. Experimentally, the water indicates that the number of H+ and OH− ions is equal. It has also been established that the product of the two concentrations equals a constant ‘K.’ This constant’s value was discovered to be between 10 and 14.
.
As Figure 1 shown, pH ranges from 0 to 14, with 7 being neutral. pHs less than 7 are acidic while pHs greater than 7 are alkaline (basic).
3.1. Na2SiO3 Sample
The initial sample consisted of a natural material of silica SiO2 with a micro-thermal fusion technology, which works to generate a bio-field of up to 80 cm. Our research is concentrating about the physical effect on oxonium ions in any matrix and thru this Field we make change into alkaline state due to the active bride which have been created between silica and sodium atoms. This contribution helping to raise the pH of water to up to 8. In order to obtain a higher purity, more powerful acids such as hydrofluoric acid can be used in addition to thermal shocks. After the refining process the sand is dry, and some applications require it to be milled to produce very thin materials, called silica flour as depicted in Figure 2.
Quartz can also be converted to crystallite in a rotary kiln operating at elevated temperatures, with the inclusion of catalysts. Also, some applications require that quartz be melted in electric arc furnaces to be cooled and then milled to produce silica magma the results of this sample coined in Table 1.
4. Evaluation Methodology and Results
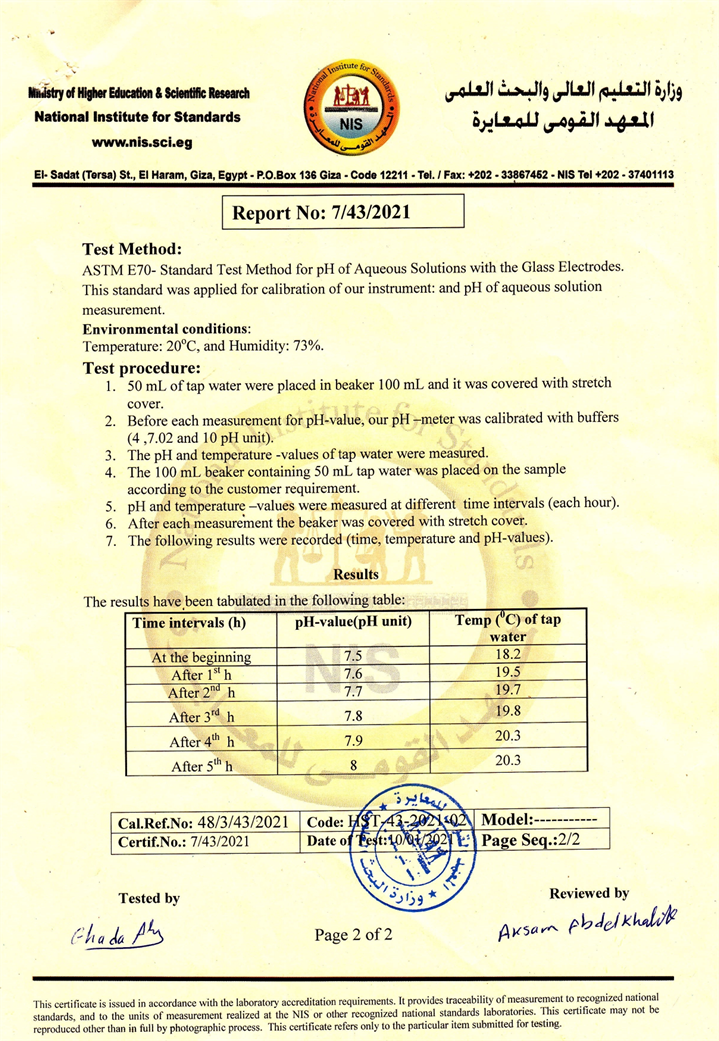
The solution was then assayed for increasing the pH value using the ASTM E70-Standard test method. This standard was applied for calibration of our instrument, and pH of aqueous solution measurement. The test environment was at 20˚C, 73% humidity [12].
4.1. Evaluation Procedure
To assess whether the pH values are increased and received, the evaluation criteria
![]()
Figure 2. The forms of Na2SiO3 Sample used in this investigation.
were as follows:
1) In a 100 mL beaker, 50 mL of tap water was put and sealed with a stretch cover.
2) Our pH-meter was calibrated with buffers before each measurement of pH value (4, 7.02, and 10 pH unit).
3) The water temperature was measured as well as the pH value.
4) The 100 mL beaker containing the 50 mL water was placed on the samples in Figure 2 to measure the effect of the physical field on raising the pH value.
5) The temperature of the water and the pH was measured every hour (The beaker is re-covered with stretch material after each measurement) without any interference in the water except that it is installed on the base that contains the manufactured material in this study, the result of this experiment is shown in Figure 3.
The experiment lasted about five hours. Both the temperature and the pH at the beginning and until the end of the experiment were measured accurately, as shown in Figure 3, to ensure the neutrality of the measurements was carried out through the National Institute for Standard in the Arab Republic of Egypt (Appendix A).
To ensure the validity of the results, the tests were repeated according to the previous conditions in the laboratories of the Drinking Water and Wastewater Authority, Egypt (Appendix B). The results showed that the pH levels increased by using the substance in a physical field, as shown in Figure 4.
Through the previous results, we find that the pH levels of water have increased from the neutral position 7 to the alkaline state 8 and this is through a vital field without direct intervention on the water, and these results are unique in the field of improving water quality, and they have direct applications on agriculture and improving the quality Drinking water and fish farming.
4.2. Radiation Evaluation
To indicate whether the manufactured material (Silica SiO2) contains radioactive or null elements. The evaluation method and procedures are summarized in Table 2.
![]()
Figure 3. The results of the pH value over time (first experiment).
![]()
Figure 4.The results of the pH value over time (Second experiment).
![]()
Table 2. Radiation evaluation method and procedures.
The results from the previous analysis, which was carried out by an independent and charitable body at the National Institute for Standard in Egypt, indicated that the manufactured material does not contain any radioactive elements as shown in Appendix C.
Through the previous results, I find that Na2SiO3 can raise the pH levels and These results are consistent with Joni et al. (2018; 2020) [13] [14] as they concluded that “Precipitated silica scale-up can be produced by optimizing the sodium silicate (Na2SiO3) precursor with additional HCl as precipitant by direct mixing method at pH 7 at temperature 25˚C.” This, in turn, helps to raise the pH of adding it to the water.
The current results of our study are unique in that they raise the pH physically without direct interference with water, which helps protect the water from any chemical effects.
5. Conclusion
The pH value will be used as a standard to measure the properties of water. It is a measure of the concentration of hydrogen ions in a solution, which is the negative logarithm of the actual hydrogen ion concentration, meaning that the hydrogen ion concentration decreases with increasing the pH, and that the difference of one acidity unit indicates a difference of ten times the hydrogen ion concentration. The aim of the present research was to examine a natural substance of environmental elements, extracted from silica using the thermal fusion method (developed by the authors) to increase the pH of water with an 80 cm physical field without adding any components to the water. Our research is concentrating about the physical effect on oxonium aion in any matrix and thru this field we make change into alkaline state due to the active bride which has been created between silica and sodium atoms. The solution was then assayed for increasing the pH value using the ASTM E70-Standard test method and Nal (TI) Scintillation detector 3 * 3 for radiation values. The laboratory results in two independent agencies showed an increase in the pH rates of water within five hours to reach 8, and this confirms the validity of the hypothesis based on this study, which is that the vital field of the material manufactured by the authors raises the pH rates of water from a distance. The second major finding was that the used material does not contain any radioactive elements.
Acknowledgements
The researchers extend their thanks and appreciation to Mr. Major General Sherif Fares, head of the Drinking Water Company in Sharkia Governorate, Arab Republic of Egypt, for his full support and assistance in conducting the necessary analyzes and testing. We are also pleased to thank the National Research Center and the National Institute for Standardization for conducting the necessary checks to ensure the effectiveness of the manufactured material in raising pH rates in water.
Appendix A
Appendix B

Appendix C
