The Effect of Decitabine Combined with Arsenic Trioxide on DAPK Gene and HL-60 Cell Proliferation and Apoptosis ()
Received 27 October 2015; accepted 12 December 2015; published 15 December 2015


1. Introduction
Acute Leukemia (AL) is one of the common malignant tumors in China, originating from malignant clonal hematopoietic stem cell. Acute Myelocytic Leukemia (AML) accounts for about 70% of the total number of leukemia. The disease progresses rapidly with a high mortality and the course of disease is only a few months. At present, the pathogenesis of leukemia is still not clear; therefore, investigating the pathogenesis and the application of new treatments has been the main exploration direction. DNA methylation is an epigenetic modification. The hypermethylation of the gene promoter in human leukemia is related to the cell cycle regulation, apoptosis, adhesion between cells and DNA repair [1] [2] , and the normal expression of gene silencing caused cancer. Different from gene mutations, DNA methylation is a biological process which can be reversible; therefore, the application of DNA methyltransferase (DNMT) inhibitors could make certain tumor gene demethylation, thus restoring its normal function. Aberrant methylation of promoter in carcinogenesis process is an early and frequent event, and it can be used as sensitive biomarkers of tumorigenesis.
Decitabine (DAC) as a specific DNA methylation inhibitor could pass directly into the DNA by phosphorylation, inhibit DNA methyltransferase, reverse the process of DNA methylation and further make the tumor suppressor gene re-expression because of methylation inactivation, causing hypomethylation of DNA and cellular differentiation or apoptosis to exert anti-tumor effects [3] . DAC has a good effect in a variety of malignant blood diseases, and there are few side effects [4] -[6] , especially in myelodysplastic syndrome transformed into acute leukemia [7] . DAC treatment of lymphoma and many solid tumors (such as melanoma and colon cancer) is also in the process of clinical trials [8] -[10] . Several studies have confirmed that, DAC could induce apoptosis and promote the role of differentiation in a variety of leukemia cell lines and further enhance the sensitivity of some traditional chemotherapy drugs in the cell lines, which is very promising for anticancer drugs [10] [11] .
Chinese first used arsenic agent in the treatment of leukemia. At present, the research on the treatment of AS2O3 has been deep, and the clinical curative effect is obvious, especially for the treatment of Acute Promye- locytic Leukemia (APL) induced apoptosis, and promoting differentiation of the targeted therapy effect is particularly prominent. In addition to the obvious effect of AS2O3 on APL, for other types of leukemia cells (such as HL-60 and K562) and some solid tumor cells, they have obvious effect on inhibiting proliferation and inducing apoptosis [12] [13] . AS2O3 has anti-leukemia cells, inducing apoptosis and promoting cell differentiation of triple role, but also it has demethylation [14] . So, it can be used as a kind of demethylation drugs in clinical application.
Death associated protein kinase (DAPK) is a calmodulin-regulated serine/threonine protein kinase and it is a positive regulator of apoptotic factor [15] . DAPK can participate in IFN-γ, TNF-α, P53, and other cell-mediated apoptotic signaling pathway [16] - [18] . DAPK is characterized by a conserved Ca2+/Calmodulin (CaM) regulatory domain, a serine/threonine kinase domain, and several conserved noncatalytic domains. DAPK also participates in autophagic death and caspase-independent necrotic death under certain cellular settings [19] . This broad involvement in cell death is attributed to the ability of DAPK to activate multiple death-promoting molecules and pathways [20] . Furthermore, the actin cytoskeleton-localized DAPK controls cell morphological changes associated with cell death. Research confirms that DAPK was widely expressed in normal tissues; however, the expression was relatively low in the head and neck, respiratory tract, urogenital tract and blood [18] , which was mainly because of the hypermethylation of DAPK gene promoter CpG island in tumor cells [21] - [23] .
In this study, DAC and AS2O3 of different concentrations monotherapy and two-drug combination treatment were used in HL-60 cells. CCK8 method and flow cytometry were used to detect the cell proliferation, and apoptosis. Reverse transcription polymerase chain reaction (RT-PCR) was performed to obtain the DAPK mRNA expression level in HL-60 cells and our study aimed to use it effectively in clinical application, which may provide a theoretical basis for the treatment of AML.
2. Materials and Methods
2.1. Cell Lines Culture and Grouping Type
Human acute myeloid leukemia cell line (HL-60) was purchased by the Chinese Academy of Sciences Shanghai Institutes for Biological Sciences Cell Resource Center. 20% FBS (Fetal bovine serum, FBS), penicillin (100 U/ml) and streptomycin (100 μg/ml) was included in RPMI1640 medium. Cells were cultured at 37˚C and 5% CO2 in the incubator. The medium was changed every 1 - 2 days.
There were a total of 14 experimental groups which were divided into control and experimental groups. The 14 experimental groups were as follows:
A1: DAC 20 μmol/L; A2: DAC 40 μmol/L; A3: DAC 80 μmol/L;
B1: AS2O3 1 μmol/L; B2: AS2O3 2.5 μmol/L; B3: AS2O3 5 μmol/L;
C1: A2 + B2; C2: A3 + B2;
C3: A2 + B3; C4: A3 + B3;
D1: A3 (pretreatment) + B1; D2: A3 (pretreatment) + B2; D3: A3 (pretreatment) + B3;
E: control group.
2.2. The Effect of DAC and AS2O3 of HL-60 on Cell Proliferation by CCK8
HL-60 cells of logarithmic growth phase were adjusted at the concentration of 1 × 105/ml and they were inoculated in 96-well plates with volume of 100 μl in each well. Various concentrations of the DAC or AS2O3 was added to each well with a final volume of 200 μl, and set up a blank control wells, each concentration was set with five wells. After incubated for 24 h, 48 h, 72 h with 5% CO2 at 37˚C in incubator, a total 20 μl CCK8 was added into each well dark and cultured in an incubator for 2 h. The microplate reader was used to measure the absorbance value in each well at a wavelength of 450 nm, and each mean value of triplicate wells was taken. OD values could reflect the number of survival cell, proliferation curve was drawn, and the inhibition of cell proliferation was calculated. The experiment was repeated for three times. The calculation formula was as follow:
Inhibition of cell proliferation rate (%) = (OD control group − OD experimental group)/(OD control group − OD zero set group) × 100%
2.3. Annexin V/PI Staining Detected the Apoptosis of HL-60 Cell
HL-60 cells of logarithmic growth phase were adjusted at the concentration of 1 × 106/ml, and they were inoculated in 6-well plates with volume of 1 ml in each well. Different concentrations of the DAC or AS2O3 were added into each well with a final volume of 2 ml. After incubated for 24 h, 48 h, 72 h at 37˚C in 5% CO2 incubator, cells were strained by Annexin V/PI.
2.4. DAPK Gene Amplification Using Real-Time PCR
Total RNA was extracted and the reverse transcription reaction was carried out to obtain cDNA. The RT-PCR was performed in a total volume of 20 μl containing 2 μl cDNA, 10 μl 2 × All-in-One qPCR Mix, 1 μl forward primer, 1 μl reverse primer, 2 μl 1st strand cDNA and 4 μl deionized water. The RT-PCR conditions were as follows: 40 cycles at 94˚C for 5 min, 94˚C for 30 s, 55˚C for 30 s, 72˚C for 1 min. The relative expression levels were detected by fluorescence ration PCR instrument and calculated using the 2−ΔΔCt comparative CT method.
2.5. Statistical Analysis
SPSS 13.0 software was used for statistical analysis. All the data were expressed by the mean value of three independent experiments and the experimental results were showed as mean ± standard deviation (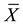 ± S); T-test was used between two groups, analysis of variance was used to compare multiple groups, P < 0.05 was considered to be statistically significant difference.
± S); T-test was used between two groups, analysis of variance was used to compare multiple groups, P < 0.05 was considered to be statistically significant difference.
3. Results
3.1. CCK8 Methods Detecting the Effect of Cell Proliferation
HL-60 cells were treated with different concentrations of DAC (20 μmol/L, 40 μmol/L, 80 μmol/L), AS2O3 (1 μmol/L, 2.5 μmol/L, 5 μmol/L) monotherapy for 24 h, 48 h, 72 h, along with the extension of the drug concentration and time, proliferation inhibition rate had gradually increased. For DAC, the cell proliferation inhibition rate of HL-60 cells had increased from (10.97 ± 1.09)% to (61.95 ± 1.24)% (Figure 1(a)), while cell proliferation inhibition rate of AS2O3 monotherapy had increased from (13.81 ± 3.09)% to (84.18 ± 2.94)% (Figure 1(b)). The difference between the treatment groups were statistically significant (P = 0.00). Monotherapy of DAC, AS2O3 could inhibit the proliferation of HL-60 cells, and it was time- and dose-dependent.
The cell proliferation inhibition rate had increased from (43.22 ± 4.78)% to (93.12 ± 3.71)% when DAC (40 μmol/L, 80 μmol/L) was combined used with AS2O3 (2.5 μmol/L, 5 μmol/L). Compared with monotherapy group, the proliferation inhibition rate was significantly increased and had statistically significant difference (P
![]()
Figure 1. (a) The inhibition rates of HL-60 cells treated by different concentrations of DAC (20 μmol/L, 40 μmol/L, 80 μmol/L) for 24 h, 48 h, 72 h; (b) The inhibition rates of HL-60 cells treated by different concentrations of AS2O3 (1 μmol/L, 2.5 μmol/L, 5 μmol/L) for 24 h, 48 h, 72 h; (c) The inhibition rates of HL-60 cells treated by DAC (80 μmol/L) combined with AS2O3 (5 μmol/L) for 24 h, 48 h, 72 h; (d) After DAC (80 μmol/L) pretreatment, the inhibition rates of HL-60 cells treated by AS2O3 (2.5 μmol/L) for 24 h, 48 h, 72 h.
= 0.00). DAC and AS2O3 had a synergistic effect on HL-60 cell proliferation (Figure 1(c)). DAC (80 μmol/L) was firstly used for pretreatment, then, different concentrations of AS2O3 (1 μmol/L, 2.5 μmol/L, 5 μmol/L) were used for 24 h, 48 h, 72 h. It was found that cell proliferation inhibition rate had increase from (31.8 ± 1.32)% to (91.44 ± 0.78)% and compared with AS2O3 mono-therapy group, it could significantly enhance inhibiting cell proliferation (Figure 1(d)).
3.2. The Effect of DAC and AS2O3 on Apoptosis of HL-60 Cell
After HL-60 cells were treated with different concentrations of DAC (20 μmol/L, 40 μmol/L, 80 μmol/L) , AS2O3 (1 μmol/L, 2.5 μmol/L, 5 μmol/L) monotherapy for 24 h, 48 h, 72 h, the apoptosis rate was gradually increased. For DAC, the apoptosis rate of HL-60 cells had increased from (8.0 ± 1.05)% to (36.6 ± 1.35)%, and HL-60 cell apoptosis rate of AS2O3 monotherapy had increased from (10.2 ± 1.35)% to (45.3 ± 2.35)%. The difference between the treatment groups were statistically significant (P = 0.00). Monotherapy of DAC, AS2O3 could induce apoptosis of HL-60 cells, and it was time- and dose-dependent (Figure 2).
The cell apoptosis rate had increased from (19.6 ± 0.56)% to (62.1 ± 0.71)% when DAC (40 μmol/L, 80 μmol/L) was combined used with AS2O3 (2.5 μmol/L, 5 μmol/L). Compared with monotherapy group, apoptosis rate was significantly increased and had statistically significant difference (P = 0.00). DAC and AS2O3 had an synergistic effect on HL-60 apoptosis rate. DAC (80 μmol/L) was firstly used for pretreatment, then, different concentrations of AS2O3 (1 μmol/L, 2.5 μmol/L, 5 μmol/L) were used for 24 h, 48 h, 72 h. It was found that apoptosis rate had increase form (18.8 ± 1.32)% to (71.4 ± 0.79)% and compared with AS2O3 monotherapy group, it could significantly enhance inducing cell apoptosis (Figure 2).
3.3. The DAPK mRNA Expression Level of HL-60 Cells after Treated with DAC and AS2O3
After HL-60 cells were treated with different concentrations of DAC (20 μmol/L, 40 μmol/L, 80 μmol/L), AS2O3 (1 μmol/L, 2.5 μmol/L, 5 μmol/L) monotherapy for 72 h, mRNA expression of DAPK was gradually increased. For DAC, the mRNA expression of DAPK had increased from (1.5 ± 0.34) to (3.2 ± 0.56) (Table 1),
![]()
Figure 2. Apoptosis rates of HL-60 cells treated by different concentrations of DAC and AS2O3 for 48 h, detected by flow cytometry ((a) control; (b): DAC 20 μmol/L; (c): DAC 40 μmol/L; (d): DAC 80 μmol/L; (e): AS2O3 1 μmol/L; (f): AS2O3 2.5 μmol/L; (g): AS2O3 5 μmol/L; (h): DAC 40 μmol/L + AS2O3 2.5 μmol/L; (i): DAC 80 μmol/L + AS2O3 2.5 μmol/L; (j): DAC 40 μmol/L + AS2O3 5 μmol/L; (k): DAC 80 μmol/L + AS2O3 5 μmol/L; (l): DAC 80 μmol/L (pretreatment) + AS2O3 1 μmol/L; (m): DAC 80 μmol/L (pretreatment) + AS2O3 2.5 μmol/L; (n): DAC 80 μmol/L (pretreatment) + AS2O3 5 μmol/L).
![]()
Table 1. The expression level of DAPK mRNA in HL-60 cells after treated by different concentrations of DAC for 72 h.
*P = 0.00, compared among each group.
and mRNA expression of DAPK of AS2O3 monotherapy had increased from (1.2 ± 0.50) to (3.0 ± 0.17) (Table 2).
The mRNA expression of DAPK had increased from (3.6 ± 0.47) to (5.2 ± 0.25) when DAC (40 μmol/L, 80 μmol/L) was combined used with AS2O3 (2.5 μmol/L, 5 μmol/L) (Table 3). Compared with monotherapy group, mRNA expression of DAPK was significantly increased and had statistically significant difference (P = 0.00). DAC and AS2O3 had a synergistic effect on mRNA expression of DAPK. DAC (80 μmol/L) was firstly used for pretreatment, then, different concentrations of AS2O3 (1 μmol/L, 2.5 μmol/L, 5 μmol/L) were used for 72 h. It was found that mRNA expression of DAPK had increase form (2.0 ± 0.35) to (4.5 ± 0.18) and compared with AS2O3 monotherapy group, it could significantly enhance mRNA expression level of DAPK (Table 4).
4. Discussion
Death-associated protein kinase (DAPK) is a tumor suppressor gene, apoptosis system is activated at the beginning of apoptosis, and the expression levels increase with increased apoptosis. Study has shown that apoptosis process is closely related to the development and tumor metastasis or DAPK gene expression levels. DAPK can activate the classical p53-caspase apoptosis pathway or caspase-independent apoptosis in multiple apoptotic pathways, it is considered likely to be multiple signal DAPK confluence of apoptosis [24] [25] . It has been confirmed that the former after DAC treatment, with the resumption of DAPK gene expression, apoptosis rate is also increased [26] . The methylation of DAPK gene promoter is an important indicator for leukemia prognosis [26] -[28] .
![]()
Table 2. The expression level of DAPK mRNA in HL-60 cells after treated by different concentrations of AS2O3 for 72 h.
*P = 0.00, compared among each group.
![]()
Table 3. The expression level of DAPK mRNA in HL-60 cells after treated by different concentrations of DAC combined with AS2O3 for 72 h.
*P = 0.00, compared with the single drug group (C1: DAC 40 μmol/L + AS2O3 2.5 μmol/L; C2: DAC 80 μmol/L + AS2O3 2.5 μmol/L; C3: DAC 40 μmol/L + AS2O3 5 μmol/L; C4: DAC 80 μmol/L + AS2O3 5 μmol/L).
![]()
Table 4. After DAC (80 μmol/L) pretreatment, the expression level of DAPK mRNA in HL- 60 cells after treated by different concentrations of AS2O3 for 72 h.
*P = 0.00, compared with the single drug group of AS2O3 and each treatment group.
Decitabine (DAC) is a cytosine analogue, as a new generation of specific inhibitor of DNA methylation, it can play DNA demethylation, and activate silent tumor suppressor genes. The previous studies have shown that after treated with DAC for 72 hours, the proportion of HL-60 cells in G0/G1 phase had increased, and the proportion of HL-60 cells in S phase had reduced. This indicated that the DAC can inhibit the cell proliferation. Soncini M et al. [29] had found that DAC can induce NB4 cell apoptosis, and its mechanism may be related to activation of the extrinsic apoptosis pathway. The mechanisms that AS2O3 induced apoptosis include: accelerated the expression level of p53 gene, down-regulated the expression level of bcl-2 gene, activated Caspase pathway and reducted membrane potential of mitochondrial [30] [31] . Some researchers have shown that AS2O3 could significantly inhibit HL-60 cell proliferation and induce apoptosis. Its mechanism may be related to the inhibition of extracellular signal-regulated kinase (ERK) signal transduction pathway.
In this study, a different concentration of DAC (20 μmol/L, 40 μmol/L, 80 μmol/L) and AS2O3 (1 μmol/L, 2.5 μmol/L, 5 μmol/L) treatment was selected for HL-60 cells to compare the DAC and AS2O3 monotherapy and combination treatment. CCK8 method was used to detect the cell proliferation and the results showed that effective inhibition of cell proliferation was time- and dose-dependent. After the two-drug combination, the inhibition rate was significantly enhanced, which indicated a synergistic effect of the two drugs. Beside this, the cell apoptosis and mRNA expression level detection also should be the obvious synergistic effect between DAC and AS2O3. This study found a relative low DAPK gene expression in HL-60 cells before drug intervention. DAPK gene significantly increased after demethylation and cell proliferation was inhibited, the apoptosis rate increased significantly, thus the reactivation of DAPK gene may be one of the mechanisms DAC induced apoptosis in HL-60 cells.
5. Conclusion
In summary, DAC or AS2O3 single drug could inhibit HL-60 cell proliferation and induce cell apoptosis, and it had a time- and dose-dependent manner. At the same time, we found that the inhibition of proliferation and the apoptosis effect of DAC combined with AS2O3 group were more obvious than single drug group, suggesting that the two drugs have a synergistic effect. The DAC pretreatment could enhance the sensibility of AS2O3 and increase the expression level of DAPK gene. Our study may provide a significant theoretical basis for the study in HL-60 cell proliferation and apoptosis.