Kinetics and Stoichiometry of the Reduction of Hydrogen Peroxide by an Aminocarboxylactocobaltate(II) Complex in Aqueous Medium ()
1. Introduction
Hydrogen peroxide is involved in all of life’s vital processes as it is versatile in its uses and applications. Its varied utility ranges from Fenton’s reagent [1] , bleaching [2] , catalytic activity [3] , oxidant for industrial chemical effluents [4] [5] , biological and medical applications [6] [7] . Its current use in industrial oxidation processes has offered environmental advantages. The kinetics and mechanisms of its decomposition by transition metal complexes, which demonstrates the importance and role of specific free radical species [8] , have received considerable attention [3] [9] - [11] . The reactions have been studied under acidic [9] [10] and basic [11] media and the mechanisms for the decomposition reaction established in addition to other kinetic parameters which are calculated. Furthermore, Schmitz and Furrow [12] used iodate reduction by hydrogen peroxide to demonstrate Bray-Liebhafsky and Briggs-Rauscher oscillatory reactions and Yong, et al. [13] studied the reduction of the latter at Au (100) electrode.
Kinetics and redox reaction involving H2O2 and transition metal complexes has also attracted the interest of other workers [14] - [16] . This study is a further effort in the general interest in the redox reaction of this versatile substrate. It is hoped that the data from this study will be an integral part in shedding more light about the mechanism of reaction of the oxidant in terms of outer- and inner-sphere mechanism.
2. Experimental
The [CoHEDTAOH2]− complex was prepared according to the method of Mansour [17] and was characterized using UV/Visible. The UV/Visible spectrum of [CoHEDTAOH2]− was scanned between wavelength ranges of 350 - 600 nm and gave λmax of 510 nm.
Standard solution of perchloric acid (Sigma-Aldrich) was prepared by diluting concentrated acid (70%, specific gravity 1.67) using distilled water. The solution was standardized titrimetrically using B4O7Na2∙10H2O as primary standard and methyl red indicator. NaClO4 (GPR) was used to maintain the ionic strength, (I). A stock solution of sodium ethanaote was prepared by weighing known amount and dissolving in known volume of distilled water.
2.1. Stoichiometric Study
The stoichiometry of the reaction was determined by spectrophotometric titration using the mole ratio method [18] -[22] . The concentration of [CoHEDTAOH2]− was kept constant at 0.01 mol dm−3 while that of [H2O2] was varied at least 10 folds below and above. The reactions were allowed to go to completion at constant [H+] and ionic strength (0.5 mol dm−3). The absorbances of the solutions were taken at 510 nm. The mole ratio was determined from the plot of absorbance versus mole ratio [CoHEDTAOH2]−: [H2O2] (Figure 1).
![]()
Figure 1. Variation of absorbance with mole ratio for the [CoHEDTAOH2]−-H2O2 reaction.
2.2. Kinetic Study
The rates of the reactions were studied under pseudo-first order condition by observing the change in absorbance of [CoHEDTAOH2]− at 510 nm on El UV/Vis digital spectrophotometer model 371 at constant [H+], temperature and ionic strength. The plots of log (A∞ - At) versus time were made (Figure 2). From the gradient of the plots the pseudo-first order rate constants, k1, were determined. The second order rate constant (k2) was determined as k1/[Oxidant]. The results are presented in Table 1. The influence of [H+] and the effect of ionic strength on the rate were investigated within the ranges 0.015 - 0.06 mol dm−3 and 0.1 - 1.25 mol dm−3 (NaClO4) respectively while all other conditions were kept constant.
2.3. The Effect of Added Anion on the Reaction Rate
The concentration of all other reactants were kept constant at I = 1.0 mol dm−3 (NaClO4). The effect of added CH3COO− on the reactions was investigated for [CH3COO−] = (2.0 - 8.0) × 10−3 mol dm−3.
2.4. Free Radical Test
About 5 cm3 of acrylamide solution was added to a partially oxidized reaction mixture containing various concentrations of oxidant, reductant and hydrogen ion. This was followed by addition of a large excess of methanol. The same treatment was applied to solution of oxidant and reductant separately. This served as control.
2.5. Product Analysis
The UV/Visible spectrum of the reaction product scanned between wavelength ranges of 350 - 600 nm gave λmax at 380 and 536 nm which agrees with literature value of λmax for Co(III) product [23] -[25] .
3. Results and Discussion
3.1. Stoichiometry
The spetrophotometric titrations showed reductant-oxidant ratio of 2:1 represented by the stoichiometric equation
2[CoHEDTAOH2]− + H2O2 + 2H+ → 2[CoHEDTAOH2] + 2H2O (1)
![]()
Figure 2. Pseudo―first order plot for [CoHEDTAOH2]−-H2O2 reaction.
![]()
Table 1. Pseudo―first order and second order rate constants for the reaction of [CoHEDTAOH2]−-H2O2, at [CoHEDTAOH2]− = 1.0 × 10−3 mol dm−3, [H+] = 0.015 mol dm−3, I = 0.5 mol dm−3 (NaClO4), T = 28˚C and λmax = 510 nm.
Stoichiometry 2:1 obtained in this reaction is generally common to reaction involving H2O2 [14] .
3.1.1. Kinetic Study
The pseudo-first order plot was linear for about 70% of the reaction time (Figure 2) and the slope of 1.12 was obtained from the logarithmic plot of k1 versus [H2O2]. The result shows the reaction is first-order in [CoHEDTAOH2]− and [H2O2] respectively and second order overall. Similar orders have been reported for H2O2 and [CoHEDTAOH2]− reactions by earlier workers [14] [15] .
3.1.2. Acid Dependent Study
The inverse acid dependence of reaction rate obtained in this study implies a protolytic equilibrium involving a deprotonated species. Plot of k2 versus [H+]−1 (Figure 3) was linear with zero intercept implying that the deprotonated specie is the reactive form [26] . Inverse acid dependence has been reported for H2O2 reactions [14] -[16] . This nature of acid dependence differs from that of Baneejee and Pujari [14] where two terms rate law, one which is dependent on acid and the other which is acid independent was established. As a weak acid, H2O2 undergoes the dissociation equilibrium
 (2)
(2)
The dissociation constant is 2.6 × 10−12 mol dm−3 at 30˚C [14] [27] . Under the condition of this reaction, [H2O2] = 1.20 mol dm−3, the [H+] 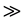 dissociation constant, the
dissociation constant, the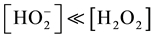 . So under the reaction condition the oxidant is predominantly present in the molecular (H2O2) form. The insensitive of rate of reaction to changes in the ionic strength of the medium further confirm that an uncharged specie is likely to be one of the reactant species as insensitivity of reaction to change in ionic strength as obtained in this study is consistent with reaction involving interaction of charged and uncharged reactants [28] .
. So under the reaction condition the oxidant is predominantly present in the molecular (H2O2) form. The insensitive of rate of reaction to changes in the ionic strength of the medium further confirm that an uncharged specie is likely to be one of the reactant species as insensitivity of reaction to change in ionic strength as obtained in this study is consistent with reaction involving interaction of charged and uncharged reactants [28] .
3.1.3. Detection of Free Radicals
To determine whether presence of free radical was important in this reaction, acrylamide solution was added to a partially oxidized reaction mixture followed by excess methanol. Gelation of the reaction is taken as evidence for the presence of free radicals [29] . The test proved negative for the titled reaction. Banerjee and Pujari [14] however implicated free radical for the reaction of these substrates.
3.1.4. Michaelis-Menten Plot
Michaelis-Menten plot (Figure 4) was linear with zero intercept, suggesting that the activated complex is composed of reactants whose centers are not linked by a bridging ligand, consequently, the reaction is susceptible to ion catalysis [30] . The added CH3COO− as expected catalyzed the reaction thereby reinforcing the result from Michaelis-Menten plot. In this paper, the kinetics and stoichiometry of [CoHEDTAOH2]−-H2O2 reaction in aqueous medium was studied. Evidences in this paper showed that the reaction is occurring through the outer- sphere mechanism. The choice of the mechanism is supported by,
![]()
Figure 3. The plot of k2 versus [H+]−1 for the [CoHEDTAOH2]−-H2O2 reaction.
![]()
Figure 4. Michaelis-Menten plot for the [CoHEDTAOH2]−-H2O2 reaction.
1) The Michaelis-Menten plot gave zero intercept.
2) Catalysis of reaction by added anion
3) The rate law obtained which is first order in each reactant specie. Rate law of this nature is known to be requisite for reactions occurring by the outer-sphere mechanism [31] .
Although the equilibrium in Equation (2) has been established for hydrogen peroxide reactions, the nature of acid dependent obtained in this study coupled with the insensitivity of the reaction to change in ionic strength; envisaging an activated complex composed of anions ([CoHEDTAOH2]− and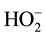 ) is not supported by experimental result in this study. The mechanistic scheme below which involves a deprotonation of the complex specie [32] explains the observed experimental data:
) is not supported by experimental result in this study. The mechanistic scheme below which involves a deprotonation of the complex specie [32] explains the observed experimental data:
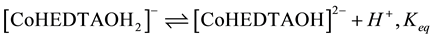 (3)
(3)
 (4)
(4)
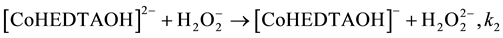 (5)
(5)
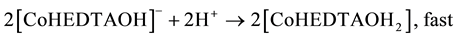 (6)
(6)
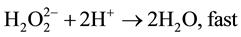 (7)
(7)
Given that
 (8)
(8)
![]() (9)
(9)
![]() (10)
(10)
which conforms to the experimental rate law, k = k1Keq.
4. Conclusion
The kinetics and stoichiometry of the reduction of H2O2 by [CoHEDTAOH2]− complex was studied in acidic medium at constant ionic strength (0.5 mol dm−3) using NaClO4. The mole ratio of reductant:oxidant is 2:1. The reaction was first order with respect to [H2O2] and [CoHEDTAOH2]− and displayed negative acid dependence. The plot of acid dependent rate constant versus [H+]−1 was linear starting from the origin. The reaction was insensitive to changes in ionic strength of the medium, indicative of composition of activated complex by charged and uncharged reactant species. The zero intercept obtained from the Michaelis-Menten plot showed absence of detectable intermediates. Evidences from this study showed that the reaction occurred through the outer-sphere mechanism and a plausible mechanistic scheme which explained the experimental data was proposed.
Acknowledgements
The authors are grateful to the Federal College of Education (now Federal University of Education) Zaria for work-study leave granted to Onu, A.D.
NOTES
*Corresponding author.
#Of blessed memory.