1. Introduction
Plant essential oils are aromatic oily liquids, having highly specific volatile odor or flavors and show broad- spectrum antimicrobial activity against diverse groups of pathogens [1] . These are obtained from various plant parts such as flowers, buds, seeds, leaves, twigs, barks, woods and roots [2] . More than 60 families of angiosperms mainly Lamiaceae, Rutaceae, Geraniaceae, Apiaceae, Aspetaceae, Lauraceae, Fabaceae, and Poaceae possess essential oils in different plant species. Essential oils are complex mixtures of diverse chemical constituents such as lectins, polypeptides, alkaloids, phenols, quinines, flavones, flavonoids, terpenes, tannins, coumarins, benzene derivatives, various hydrocarbons and straight chain compounds. Plant essential oils multiple biological activity such as antibacterial [3] , antifungal [4] , anti-cancer [5] [6] and anti-oxidant [7] . Essential oils are obtained in pure form or a complex mixture of several components without making any change in their chemical composition [8] . These are used for a wide variety of purposes [9] such as flavoring, perfuming [10] aromatherapy and food preservation [11] - [13] . These are obtained by fractionation or rectification and steam distillation of various plant parts in Clevenger apparatus [14] . Essential oils possess mixed functional groups, too complex in their structure and are highly volatile at a very low temperature. Due to high volatility essential oils easily spread in the environment and medium. These act more efficiently against drug resistant microbes in culture medium due to their fast diffusion and volatile action. Treatment with plant essential oils shows least residual effect in the body, but these severely inhibit growth and metabolism of a variety of infectious pathogens mainly microbes. These are also used alternative medicine in aromatherapy for treatment of cancer, disease pathogens and dermal infections [15] [16] .
Saunf (Foeniculum vulgare) is an annual or biennial plant belonging to family Umbelliferae family. It has sparse, fine, feathery leaves and pinkish/white flowers, which are followed by green seeds. Fennel oil has a sweet, spicy, warm smell, is nearly colorless to pale yellow and has a watery viscosity. Fennel seeds are rich source of essential oil which is extracted by steam distillation and yields 0.8% - 1.0% oil. Due to strong flavor, essential oil extracted from seeds is used for medicinal purposes such as aphrodisiac, antispasmodic, carminative, depurative, deodorant, digestive, fungicidal, lipolytic, stimulant and stomachic substance. It is used in aromatherapy and helps to ease the mind and fight fatigue. Saunf oil application warms and calms the digestive system, relieves rheumatism and arthritic pain, muscular spasms and detoxifies the body [9] . It is also used for massage, relieve mental tensionand fatigue. Oil vapors provide freshness and show warming effect on the stomach, relieve wind and cramps, and revitalizing the glandular system. Fennel is famous worldwide as a spice and is regularly used in vegetables, and has multiple medicinal properties as well, such as its digestive and stomachic properties. Fennel seeds are often used as a spice in Indian cuisine and cooked well till the flavor blends with the dish. It has lemony, citrusy flavor when crushed, and it contains pinene and linalool terpenes. Fennel seeds are at their best when used fresh. Often, it is dry roasted and grounded before being added to a dish. Fennel oil improves appetite, regulates endocrinal secretions, cures nausea and eliminates vomiting as a symptom of many conditions. In the present study, essential oil (E.O.) from Foeniculum vulgare was isolated from ripe seeds and screened for its antimicrobial activity in vitro by applying various bioassays. For evaluation of antimicrobial susceptibility potential various tests and growth inhibitory bioassays were conducted by setting controls and treatments done in seven bacterial strains (i.e. Escherichia coli, Bacillus cereus, Klebsiella pneumoniae, Lactobacillus acidophilus, Staphylococcus aureus and Streptococcus pneumoniae and Micrococcus luteus) and three isolates of fungi (i.e. Candida albicans, Aspergillusniger, Rhizopus stolonifer). MIC, MBC, MFC, inhibition zone diameters (DIZ) were determined in bacterial and fungal strains were determined in presence of each varying concentrations of essential oil. Antimicrobial susceptibility obtained in Fennel essential oil was compared with broad-spectrum antibiotics drugs.
2. Experimental
2.1. Instrumentation
Gas-Chromatography-Mass Spectrometry (GC-MS)
GC/MS analyses of Foeniculum vulgare E.O. was carried out on a Shimadzu GC-MS-QP2010 apparatus equipped with a −5 column (60 m × 0.25 mm i.d., film thickness 0.25 µm). Helium was used as carrier gas at a constant column flow 1.2 ml/min at 173 kpa inlet pressure and injector volume was 1.0 µL. The test was performed according to a set temperature programming which was maintained from 100˚C to 200˚C with constant rise of 5˚C/min and then held isothermal at 200˚C for 6 min. Further, the temperature was increased by 10˚C/min up to 290˚C and again held isothermal at 290˚C for 10 min. The injector and ion source temperatures were 270˚C and 250˚C, respectively. The crude and active bands (AB-1) and (AB-2) (2 mg/ml) were dissolved in methanol (HPLC grade, Merck, India) and are injected with a split ratio of 1:10. Mass spectra were taken at 70 eV; a scan interval of 0.5 s and fragments from 40 to 950 Dalton. The identification of the essential oil components was based on the comparison of their relative retention time (tR) and mass spectra with those of commercial standards (for the main components) and on the GC retention indices (RT) determined rel. to n-alkanes (C8 - C32). The final confirmation of constituents was made by computer matching of the mass spectra of peaks with the Wiley and National Institute Standard and Technology (NIST) libraries mass spectral database. The relative percentages of the essential oil constituents were calculated from the GC peak areas. Further, quantitative analysis was performed by means direct peak area intention technique based on the total ion chromatogram (TIC).
2.2. Plant Material
The seeds of Foeniculum vulgare were purchased from local market at Gorakhpur, Uttar Pradesh, India. The identification of plant material was made by plant taxonomist. A voucher specimen is deposited for further confirmation in the departmental laboratory.
2.3. Extraction and Isolation of Essential Oil
Seeds of Foeniculum vulgare were grounded by using domestic mixer and powdered material was hydro-dis- tilled in Clevenger apparatus continuously for 5 hrs to yield essential oil [13] . The crude powder was extracted with pure methanol twice and dried residue was dissolved in known volume of fresh solvent (w/v) before testing the antimicrobial activity.
2.4. Identification of Major Constituents
The chemical constituents of essential oil from Foeniculum vulgare are listed in Table 1. Thirty six components were identified by using GC representing 99.98% of the oil. The main constituents of essential oil were identified 9-octadecenoic acid (18.56%), 8Z)-14-methyl-8-hexadecenal (7.75%), pentad ecanecarboxylic acid (4.25%), o-benzenedicarboxylic acid (14.47%), 1,3,3-trimethyl-2-vinyl-1-cyclohexene (10.77%), 2-methyl-3-oxoestran- 17-yl acetate (5.46%), 1H-benzocycloheptene (10.71). The dominant components are 2-hydroxy-1-(hydrox- ymethyl) ethyl ester (1.84%), and 9-octadecenoic acid (18.56%) but in different ratios [Figure 1, Table 1] together with few compounds found in minor concentrations. Compounds were identified by comparison of retention time and MS peaks. Both formula and molecular weights were established in each case.
2.5. Determination of Antimicrobial Susceptibility
2.5.1. Source of Microorganisms
Cultures of seven pathogenic bacterial strains each of Escherichia coli (ATCC 25922), Bacillus cereus (ATCC 11778), Lactobacillus acidophilus (ATCC 53103), Micrococcus luteus (ATCC 9341), Staphylococcus aureus (ATCC 25923), Klebsiella pneumoniae (ATCC 15380) and Streptococcus pneumoniae (ATCC 12755) were maintained in the laboratory in Luria Broth (2% w/v) regularly for four days at 37˚C before use in experiments. For experiments, a portion (100 µl) of the overnight culture was mixed in the tests and control for inoculation. For activity, testing bacterial cultures were stored at 4˚C and sub cultured after every 8th day in solid agar plates. For determination of antifungal activity of plant latex, fungal strains of Aspergillus niger MTCC 1344, Candida albicans MTCC 227, Rhizopus stolonifer MTCC 3789 were grown in the laboratory. Moreover, each test fungi was maintained in strain specific agar medium mainly Sabouroud’s Agar and Potato Dextrose Agar and its pure cultures were established by using single spore isolation technique.
2.5.2. Disc Diffusion Assay
The in vitro antimicrobial activity of Foeniculum vulgare essential oil was evaluated in vitro Agar Disc Diffusion Assay. In each assay inhibition zone diameters were measured in presence and absence of essential oil. For treatments essential oil was diluted by applying serial micro-dilution method by adding Luria Broth media separately. Six different concentrations of essential oils (1 - 32 μg) (W/V) were coated on sterile filter paper discs (Whatmann No. 1) of 6 mm size and oil impregnated discs were dried under laminar flow cabinet. Before starting experiments inoculums size was determined and adjusted to prepare a final colony number as 108 colony
![]()
Table 1. Major and minor constituents isolated from Fennel (Foeniculum vulgare) essential oil.
![]()
Figure 1. GC-MS spectrum of essential oil obtained from Foeniculum vulgare.
forming units (CFU/ml) in sterile agar plates. Bacterial inoculums were spread evenly on to the surface of agar plate by using a sterile rubber pad spreader. After which essential oil coated discs were positioned on the inoculated agar surface in the centre. Essential oil was assayed in triplicate for antibacterial activity testing. All treated and untreated plates were incubated for 24 hrs at 27˚C. DMSO was used as negative control while ampicillin was used as standard (positive control) to compare the bacterial growth and Griseofulvin was used to compare fungal growth in negative control. The radial growth of fungi was measured after 12 hours interval up to 36 hours of initial inoculation. The average percentage inhibition of growth in presence of various essential oils was calculated by using following formula,
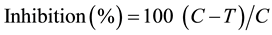
where C = diameter of fungus colony in control plates; T = diameter of fungus colony in tested plates.
2.5.3. Minimum Inhibitory Concentration Determination (MIC)
Bacterial growth inhibition was accessed in the presence of different increasing concentrations of essential oil in Luria Broth culture medium and MIC values were determined for each bacterial and fungal strain. For this purpose, essential oils were diluted in a concentration range from 48 µg/ml to 0.0058 µg/ml by using serial micro dilution method. Essential oil was added to fresh media suspension after following the serial dilutions up to 10−10. Fennel essential oil was assayed in triplicate. Before conducting experiments all the conditions for in vitro anti-microbial activity were standardized to determine MIC and MBC values. The MIC values were considered as the lowest concentration of essential oil, in which no turbidity in the culture flask was visualized after 24 hrs of incubation at 37˚C. The turbidity in the culture flasks was considered as visible growth of microorganisms. Further, it was standardized in terms of absorbance at 600 nm in a visible spectrophotometer. For determination of minimum bacterial concentration (MBC) growth inhibitory assays were performed. For this purpose inoculums’ size was adjusted to prepare a final colony number as 108 colony forming units (CFU/ml in sterile agar plates. The incubation of test and control cultures was also performed at 37˚C for 24 hours. For comparison, both negative and positive control was set and bacterial colony number was counted in all test and control discs. For comparison broad-spectrum antibiotics i.e. ampicillin was used as standard to compare the bacterial growth while Griseofulvin for comparison of fungal growth. Results were interpreted by using a standard table that relates to the degree of microbial resistance prescribed by NCCLS (National Committee for Clinical Laboratory Standards). A plot of MIC on a logarithmic scale versus zone inhibition diameters (arithmetic scale) was prepared for essential oil and antibiotic to know the susceptibility level. These plots were used to find the zone inhibition diameters corresponding to the drug concentrations and that of essential oils. The low MIC value was considered as susceptibility of essential oils/drugs to the pathogen, while high MIC value (with a small zone inhibition diameter) was considered as resistant.
2.5.4. Statistical Analysis
All statistical calculations are expressed as mean ± SE of three replicates. Data were analyzed by one way ANNOVA to locate significant variations in oil activity in various bacterial and fungal strains followed by the Duncan’s multiple range tests.
3. Results
Chemical Composition of Oil
Fennel essential oil is extracted from the seeds of coriander with the help of steam distillation. The scientific name of Fennel (Saunf) is Foeniculum vulgare. Plant origin natural products are known to have more antimicrobial activity against drug resistant microbes. This activity could act as chemical defense against pathogenic diseases. However, in the present time, both the traditional and folk medicines have been considered as alternatives of synthetic drugs for healthcare to the patients. However, for screening pharmaceutical and therapeutic potential of these natural products various bioassays are developed to detect and confirm the anti-pathogenic effects and establish a good correlation with disease pathogens. For obtaining broad-spectrum drugs, essential oils are found to be good therapeutic-targeting molecules. The present study emphasizes the composition of essential oil isolated from Foeniculum vulgare and its effect on inhibition of bacterial and fungal growths.
Few major components are identified as 2-hydroxy-1-(hydroxymethyl) ethyl ester (1.84%), and 9-octadece- noic acid (18.56) but in different ratios [Table 1] together with few compounds found in minor concentrations. The dominant components are 2-hydroxy-1-(hydroxymethyl) ethyl ester (1.84%), and 9-octadecenoic acid (18.56%) but in different ratios [Table 1] together with few compounds found in minor concentrations. Disc diffusion assays were conducted with Foeniculum vulgare essential oil to measure growth inhibition zone diameter and screen anti-microbial potential. The essential oil of Foeniculum vulgare has shown higher range of inhibition zone diameter 20.2 ± 0.18 - 26.5 ± 0.14 mm at a concentration level of 24 μg/disc. The positive control has shown diameter of inhibition zone (DIZ) ranging from 15.1 ± 0.30 - 18.8 ± 0.37 mm at concentration of 24 μg/disc. All DIZ corresponding to test organisms are mentioned in Table 2. The results of MIC obtained against all the bacterial strains have been given in Table 3. Lower MIC values presented have shown very high antimicrobial susceptibility of Foeniculum vulgare to E coli, Bacillus cereus, L. acidophilus and S. pneumoniae are in a range of 6 - 48 μg/ml.
Lower MIC values presented have shown very high antimicrobial susceptibility of Foeniculum vulgare to E coli, Bacillus cereus, L. acidophilus and S. pneumoniae are 6, 12.0, 24, 24 μg /ml respectively.
![]()
Table 2. Zone of inhibition of essential oil from methanolic extract of Foeniculum vulgare.
Values are expressed as mean ± SD (n = 3) and values followed by same letter are not significantly different at the P < 0.05; Determined by Duncan’s Multiple Range test. Positive control = Ampicillin/Griseofulvin, negative control = DMSO.
![]()
Table 3. Antimicrobial activities of essential oil from methanolic extract of Foeniculum vulgare on different microbes and their corresponding MIC.
Positive control is Ampicillin/Griseofulvin.
4. Discussion
In the present investigation, Foeniculum vulgare essential oil GC-MS analysis showed presence of 36 compounds representing more than 99.98% of the essential oil [Table 1]. The inhibition zones of the essential oils on tested organism show a significant correlation with MIC values (P < 0.05). Based on growth inhibition zone diameters obtained in tests, results were divided into three categories i.e. resistant (>7 mm), intermediate (>12 mm), and susceptible (>18 mm). Maximum growth inhibition diameter was obtained 26.5 ± 0.14 mm against Micrococcus luteus followed by 25.9 ± 0.14 mm against Lactobacillus acidophilus. Foeniculum vulgare oil has shown significantly higher growth inhibition zone diameters in Aspergillus niger 22.7 ± 0.40 mm, Candida albicans 20.5 ± 0.15 mm and 23.0 ± 0.49 mm in Rhizopus stolonifer than the broad spectrum antifungal drug griseofulvin 17.4 ± 0.30 mm [Table 2]. Similar growth-inhibition zone diameters were reported in P. aeruginosa 33.3 mm, B. subtilis 29.9 mm P. vulgaris 29.4 mm, K. pneumoniae 20.8 mm and S. aureus each [16] , Clostridium ferfringens, E. coli and Lactobacillus acidophilous [17] , Bacillus species [18] , Staphylococcus aureus [19] [20] , and Salmonella enteritidis in presence of different essential oils [21] . Coriander oil (Foeniculum vulgare) has shown 6.0 µg/ml MIC valueagainst E. coli, 12.0 µg/ml against, Bacillus cereus, 24.0 µg/ml Lactobacillus acidophilus [Table 3]. Similarly, it has shown MIC value in a range of 6 - 24 µg against Aspergillus niger, Candida albicans, Rhizopus stolonifer [Table 3]. Similar MIC 6 µg/ml was reported in allicin and diallyl sulfur compounds against Helicobacter pylori [22] . Similarly luteolin [23] , thymus [24] , phenolics [17] (2006) and Cavacrol [3] , di-terpenoids [25] isolated from various essential oils have also shown strongeranti-microbial activity against few bacteria mainly against oral pathogens [26] and Escherichia coli O157:H73. Similarly, juniper oil extracted from Juniperus communis (L) has shown strong bactericidal activity against both Gram-positive and Gram-negative bacteria with MIC values between 8% and 70% v/v [27] . Same oil has also shown stronger fungicidal activity against Candida sp. (MIC from 0.78% to 2% v/v). Sardinian juniperus essential oil was found active against foodborne pathogens and spoilage microorganism [7] . Similar, antimicrobial activity was exhibited by different Mentha species i.e. Mentha longifolia L., Mentha aquatica and Mentha piperita L. against E. coli with very low MIC value (4 µL/ml) [4] and Hypericum species such as Hypericum scabrum, Hypericum scabroides and Hypericum triquetrifolium essential oils [28] . Moreover, di-terpenoids isolated from Sagittaria pygmaea has shown antibacterial activity against Streptococcus mutans (ATCC 25175) with MIC value of 15.6 µg/ml [25] . Essential oils from Coriandrumsativum (L) [29] Cinnamomum osmophloeum [30] . Besides this, Dracocephalum foetidum essential oil also exhibited strong antibacterial activity against methicilin-resistant Staphylococcus aureus (MRSA) [31] . For comparison of antimicrobial activity of essential oils certain broad spectrum antibiotics were also tested against same bacterial strains, which have shown marginal activity orintermediate effect. In various bioassays, Foeniculum vulgare essential oil has shown very high anti-bacterial and anti-fungal activities in vitro. Similar, antimicrobial activity of essential oils of Laserpitium latifolium and L. ochridanum against one Gram-positive and three Gram-negative bacteria and two Candida albicans strains. Essential oil showed a high antimicrobial potential against Staphylococcus aureus, S. epidermidis, Micrococcus luteus, or Candida albicans (minimal inhibitory concentrations of 13.0 - 73.0 μg/ml [32] . Essential oils (E.O.s) from Zataria multiflora Boiss. (zataria) and Origanum vulgare (oregano) showed extensive antimicrobial activity in a wide range of food spoilage or pathogenic fungi, yeast and bacteria, and on hepatitis A virus [33] , Contrary to this, essential oils showed weak inhibitory activity against the Gram-positive pathogens. Essential oils of Pereskia aculeata Mill. and P. grandifolia Souza et al., 2014) [34] , Similarly Piper species: Piper abbreviatum, P. erecticaule and P. lanatum displayed weak activity towards Gram-positive bacteria with MIC values in the range 250 - 500 μg/ml. P. erecticaule oil showed the best activity on Aspergillus niger (MIC 31.3 μg/ml), followed by P. lanatum oil (MIC 62.5 μg/ml) [35] . Juniperus excelsa Bieb. leaf essential have showed high activity towards: Staphylococcus aureus, Streptococcus pyogenes and Haemophilus influenzae (MIC = 125 μl/ml). The pinene-type of essential oil showed moderate activity against Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus agalactiae, Streptococcus pyogenes, Corynebacterium spp. and Campylobacter jejuni (MIC > 50%) [36] . Essential oils isolated from the leaves of Cosmos bipinnatus showed effects against both Gram-negative and Gram-positive bacteria isolates. The MIC of Gram-positive strains ranged between 0.16 and 0.31 mg/ml while those of Gram-negative bacteria ranged between 0.31 and 0.63 mg/ml. The Gram-positive bacteria were more susceptible to the essential oil than the Gram-negative bacteria [37] .
Due to presence of volatile components, i.e. phenolic compounds in higher concentration [17] and its diffusion at room temperature fennel essential oil displayed high susceptibility against both Gram-negative and Gram-positive bacteria. In addition, it may also increase the plasma membrane permeability that results in higher leakage of fluid material from bacterial cells [32] and inhibit microbial respiration [38] . Therefore, major antimicrobial activity seems to be post diffusion action of essential oils on growth and metabolism of both the bacterial and fungal strains [39] [40] . No doubt, Saunf (Foeniculum vulgare) essential oil contains so many promising molecules, which can be used for therapeutic purposes mainly pharmacological potential [41] . Like other plant, natural products essential oils possess broad-spectrum antimicrobial activity against pathogenic microbial strains [42] . As high antimicrobial susceptibility obtained in tests incomparison to drugs, olive essential oil and its components can be used for formulation of highly active non-antibiotic drug that may be less toxic and show lesser side effects. The antibacterial activity can be attributed to effects of the combination of several components of the oil. The results indicate that the Foeniculum vulgare might be exploited as natural antibacterial agent and have application in the treatment of several infectious diseases caused by these bacteria.