Determination of Trace Amounts of Zinc, Iron and Copper by Flame Atomic Absorption after Their Preconcentration Using Sodium Dodecyl Sulfate (SDS) Coated Alumina Nanoparticles Modified with 3-Mercapto-D-Valin from Environmental Samples ()
1. Introduction
Considering biological research, the roles of some trace and ultra-trace elements in the body are very important and have diverse function [1] [2] . Some of trace elements are essential to life while others are toxic even at very low concentrations [3] [4] . Hence, their determination in industrial effluents, various water resources, environmental and biological samples are important, especially in the environment monitoring and assessment of occupational and environmental exposure to toxic metals. However, the direct determination of heavy metal ions at trace levels in real samples remains a challenging problem because of their low concentration and matrix effects even with frequently used sophisticated instrumental techniques such as inductively coupled plasma atomic emission spectrometry (ICP-AES), electrothermal atomic absorption spectrometry (ET-AAS), etc. without sample preconcentration and separation [5] [6] . Flame atomic absorption spectrometry is one of the most widespread traditional analytical techniques for the determination of trace elements, but often suffers from its low sensitivity the determination of trace quantity of heavy metals in environmental samples like natural water and other real samples of environmental interest in which they are found at very low concentrations requires the use of preconcentration methods coupled to spectroscopic methods such as ICP-AES and FAAS. The solid phase extraction (SPE) enables a wide research activity on the development of alternative methods, which are capable of highly selective for the removal of trace amounts of metal ion solution containing complicated matrices with minimal usage of organic solvents [3] [4] . The SPE can easily be adapted for FAAS to improve the detection limit and selectivity of determinations. In our laboratory using various types of sorbents including activated carbon [7] , SDS-coated alumina [8] , alumina and silica gel have gained much attention with high repeatability and large lifetime. The design of a stable and selective solid phase sorbents for separation and preconcentration of a target metal ion depends on different factors such as the nature of solid support, its surface area and activity [9] . Nano-sized γ-alumina is a kind of new functional material. It has attracted extensive attentions in recent years because of its specific physical and chemical properties such as high surface area, absence of internal diffusion resistance and high surface binding energy [10] [11] . If indeed sorption would be the key mechanism, then the substantial increase in surface area of the nanoform would increase capacities significantly. In order to improve the selectivity of the sorbent, the modification of the adsorbent is usually required. The most often used method is to load a kind of specific chelate reagent by physical or chemical procedure on the surface of this material. A new modification mode for the alumina to use in solid phase extraction works was introduced by Hiraide et al. [12] . The organic reagent is incorporated in the cores of admicelles of sodium dodecyl sulfate (SDS) surfactant attached to alumina surfaces at acidic pH. According to this improvement, new organic reagents are immobilized on surfactant-coated alumina for separation and enrichment of different metal ions [12] [13] . The goal of the present work is to establish a new preconcentration method for the trace determination of Fe(II), Zn(II) and Cu(II) by FAAS using 3-mercapto-D-valine modified alumina nanoparticles.
2. Experimental and Techniques
2.1. Instruments
A Shimadzu 680 AA, atomic absorption spectrometer was equipped with deuterium background corrector. Hollow cathode lamps were used as radiation source, and the operational conditions of the equipment were established according to the manufacturers’ recommendations for each element. The atomization was carried out in an air-acetylene flame. Infrared spectra were recorded using a Fourier transform infrared spectrometer (FT-IR, Perkin Elmer, Spectrum 100) to identify the functional groups and chemical bonding of the coated materials. Scanning Electron Microscope (SEM) was performed to measure the particle size and shape (SEM-EDX, XL30 and Philips Netherland). A Meterohm 691 pH/Ion meter (Switzerland, Swiss) with a combined glass-calomel electrode was used for the adjustment of the pH of the test solution.
2.2. Reagent and Solutions
All chemicals used in this work, were of analytical-reagent grade (Merck) and were used without further purification. Doubly distilled deionized water was used for all dilutions. All the plastic and glassware were cleaned by soaking in dilute HNO3 (1 mL HNO3 + 10 mL distilled deionized water) and were rinsed with distilled water prior to use. The element standard solutions of 1000.0 mg·L−1 of the given element were supplied by Merck. Stock solutions of diverse elements were prepared from high purity compounds. The γ-Al2O3 mesh 40 nm (X- ray analysis), spherical shape) was purchased from Plasmachem GmbH. The ligand (3-marcapto-D-valin) and SDS were purchased from Merck. The pH was adjusted by addition of 0.1 mol·L−1 nitric acid or 0.1 mol·L−1 sodium hydroxide.
2.3. Preparation of Solid Phase
The alumina nanoparticles added to nitric acid solution for 72 hours then stirred for 15 min, and then rinsed with deionized distilled water. After drying, 1.0 g of activated nano-alumina added to 30 mL of water containing 50 mg of the SDS particles, while shaking the suspension by stirrer ,the pH was adjusted to 2, then 45 mg of 3-mercapto-D-valin added to suspension and shaking for 15 min, while shaking the suspension by stirrer, the pH was adjusted to 7 with 0.1 mol·L−1 sodium hydroxide solution, afterward, Solid phase was separated from the suspension by centrifuges, then washed by deionized distilled water, after that dried and used as solid phase (sorbent).
2.4. Procedure for Preconcentration
The pH of solutions containing different amounts of the ions, was adjust to the pH 7.0 by addition of NaOH or HCl. the samples were kept contacted directly with the modified solid phase for 15 min at stirring rate of 300 rpm to effect the deposition of analytes. The retained ions were then eluted with nitric acid (5 mL of 4.0 mol·L−1). Analyte ions in the final solution were determined by atomic absorption spectrometry.
2.5. Application of Real Samples
The solid samples were digested according to procedures Ghaedi and colleagues [14] . The fruit and leaves samples of (apple and mango) were purchased from Shiraz, Iran. The samples once in the laboratory were washed with ultra pure water, and separate the edible (fruit) and nonedible (leaves) parts both samples, dried under stream of air and ground into small mesh size. A 30 g of samples was heated in silica crucible for 3h on a hot plate and the charred material was transferred to furnace for overnight heating at 650˚C. The residue was cooled, treated with 10.0 mL concentrated nitric acid and 3 mL 30% (w/v) H2O2 again kept in furnace for 2 hr at the same temperature so that no organic compound trace are left. The final residue was treated with 3 mL concentrated hydrochloric acid and 2 mL 70% (w/v) perchloric acid and evaporated to fumes, so that all the metals change to respective ions. The dissolved solid residue was filtered and its pH was adjusted at 7.0 and made up to 200 mL. Concentration was determined after caring out procedure give in Section 2.5. For tap water samples, five liters of drinking water was collected in a glass container from our research laboratory in Faculty of Chemistry, Islamic Azad University at Firouzabad city. The sample was filtered through a Millipore cellulose membrane filter of pore size 0.45 μm before its analysis. The organic content of the water sample was oxidized in the presence of 1% (w/v) H2O2 and addition of concentrated nitric acid. The pH of water sample was adjusted at 2.0 by nitric acid solution. Before preconcentration, the sample was neutralized by sodium hydroxide solution to pH 7.0. According to optimized experiment conditions and the preconcentration/separation procedure was performed. Waste-water samples were collected using a polypropylene shovel from the wastes of Shiraz Industrial Zone, and subsequently transferred to clean polypropylene bags. The organic content of the waste samples were oxidized in the presence of 30% (w/v) H2O2 and concentrated nitric acid. Volumes of 100 mL of sample were filtered through a filter paper and stored in polypropylene containers until analyzed. pH of the sample was then adjusted to 7.0 and the procedure described in Section 2.5 has been carried out [15] [16] .
3. Result and Discussion
3.1. Characteristics of Modified Alumina Nanoparticles
The adsorbent was subsequently characterized by FTIR spectral data. The FT-IR spectrum of alumina nanoparticles coated SDS modified with 3-Mercapto-D-valin has prominent bands at 1680 cm−1, 1486 cm−1, and 1387 cm−1 due to carboxylate , OH (bending) and phenolic group vibrations, respectively (Figure 1). The surface and texture morphology of alumina nanoparticles coated SDS modified with 3-Mercapto-D-valin by SEM image is illustrated in Figure 2. As shown in Figure 2, the naked alumina nanoparticles had Almost a mean diameter of 50 nm. The SEM images show that the naked alumina nanoparticles had a mean diameter of 50 nm and the modified nanoparticles had a mean diameter of 74 nm. This shows that the alumina nanoparticles have been completely coated by 3-Mercapto-D-valin. Using the ultrasonic vibrations caused that the nanoparticles have been smaller and more homogenized particles.
3.2. Variables Optimization of Preconcentration Process
3.2.1. Effect of pH for Metal ions Uptake
The influence of pH on the preconcentration of target metal ions with initial concentration of 0.30 μg·L−1 over the pH range from 3.0 to 8.0 was studied (keeping the other parameters constant). As could be seen from Figure 3 quantitative recovery (>98%) was obtained for Zn(II), Fe(II) and Cu(II) within the pH range 7.0. This may be attributed to the presence of free lone pair of electrons on nitrogen atoms, which are suitable functional sites for coordinating with the metal ions. The decrease in recovery at pH values lower than 7 can be due to protonation of the ligand and reduces the tendency for complexation with ions. Also The decrease in recovery at pH values higher than 8 can be due to the formation of Zn(OH)2, Fe(OH)2 and Cu(OH)2 [17] [18] .
3.2.2. Effects of Amounts of 3-Mercapto-D-Valin
In order to determine the concentration 3-mrcapto-D-valin requires for quantitative recoveries of all investigated metal ions, the proposed method was applied. Firstly, some works were carried out without ligands at optimum pH 7.0 and it was observed that the Zn2+, Fe2+ and Cu2+ ions were not quantitatively recovered in the absence of ligands. The studies were performed with the various amounts of 3-mrcapto-D-valin and results are presented in Figure 3. It can be seen that the recoveries of the metal ion increased with increasing concentration of 3- mrcapto-D-valin added and reached a constant value (98%) with at least 45 mg 3-mrcapto-D-valin (for 1 g alumina nanoparticles and 50 mg SDS). on this basis, studies were carried out at 3-mrcapto-D-valin concentration of 45. This concentration of ligand is enough for the separation―preconcentration procedure because of the very low level of the investigated metal ion concentration in real samples. Probably at ligand amount higher than 45 mg, due to possible formation of charged unretainable species, the amount of recovery will be reduced significantly. 45 mg of 3-mrcapto-D-valin was chosen to account for other extractable species that might potentially interference with the assaying of metal ions. The influences of the amounts of solid phase at fixed optimum values of ingredient were investigated. The results display that 1.0 g of alumina nanoparticles has the highest efficiency.
3.2.3. Effect of Amount of SDS on Metal Ions Recoveries
The influence of SDS amount on the percentage of complexed ion ad-solubilized was investigated. In the absence of SDS, metal ion retined on 3-mercapto-D-valin-alumina nanoparticles with 65% lower efficiency for ions. The retention of metal ion on hemi-micelles, which have a hydrophobic surface, was clearly dependent on analyte complex polarity. Therefore, addition of SDS is necessary. The formation of minute amounts of ad-mi- celles was essential to achieve complete ad-solubilization of chelates of these ions. At surfactant concentrations higher than about 45 mg·g−1 alumina nanoparticles, a decrease in the retention percentage of ion was observed, as result of the formation of micelles (Figure 4). The adsorption amount began to decrease when the SDS added exceeded 50 mg for 1.0 g alumina nanoparticles. It can be explained by the fact that with more SDS added, its molecules began to form micelles in the bulk aqueous solution, moreover, the micelles make the 3-mercapto-D- valin distribute into the bulk solution again. In this region, the slope of alumina nanoparticles adsorption curve is flat, which indicates that the interaction between 3-mercapto-D-valin and SDS-coated alumina nanoparticles were strong. As result, it was more difficult for SDS-coated alumina nanoparticles to desorbed 3-mercapto-D- valin into aqueous solution.
3.2.4. Effect of Type and Eluent Volume
The nature and concentration of eluting agents were found to have a significant effect on the elution process of
![]()
![]()
![]()
Figure 1. FT-IR spectra (A) alumina nanoparticles (B) alumina nanoparticles coated SDS and (C) alumina nanoparticles coated SDS modified with 3-mercapto-D-valin.
![]()
![]()
Figure 2. SEM images of purchased (A) alumina nanoparticles and (B) modified alumina nanoparticles.
the retained ions from the solid phase. As could be seen from Figure 5, the uptake of these metal ions was negligible at pH < 3.0. Therefore, the acidic eluents are the best solution for extraction of adsorbed ion. Different eluting solutions were used for elution of ion, which retained on SDS-coated alumina nanoparticles due to chelation with 3-mrcapto-D-valin. The effect of eluent volumes and concentration on the recoveries of the analytes were also investigated. Quantitative recoveries of Zn2+, Fe2+ and Cu2+ ion were obtained with 5 mL of 4 mol·L−1 HNO3 as compared to other eluents. Therefore 5 mL of HNO3 was used in the subsequent experiments.
3.2.5. Effect of Shaking Time
The shaking time is an important factor in quantitative preconcentration of the analytes by static method. In order to investigate the effect of shaking time on the extraction efficiency, extraction experiments were carried out at 2, 5, 10, 15, 20, 40 and 60 minutes time intervals (with stirring rate of 300 rpm). Results showed that the rate of uptake of analytes was quite high. After 5 min more than 90% of the analytes were adsorbed. Adsorption of analytes from the solution reached more than 97% in 10 minutes. Therefore, shaking time of 15 min was selected for further works.
3.2.6. Reusability of the Adsorbent
The prepared sorbents was subjected to several loadings with metal solution and elution with suitable eluent by
![]()
Figure 3. Effect of amount of ligand on metal ions recoveries by 5 ml nitric acid 4 M solution. The general conditions: modified Al2O3 NPs amount, 1 g; SDS amount, 50 mg; electric mixer-assisted extraction time, 15 min; metal ions concentration, 50 μg·L−1.
![]()
Figure 4. Effect of amount of SDS on recovery of metal ions by 5 ml nitric acid 4 M solution. The general conditions: modified Al2O3 NPs amount, 1 g; 3-mercapto-D-va- lin amount, 45 mg; electric mixer-assisted extraction time, 15 min; metal ions concentration, 50 μg·L−1.
![]()
Figure 5. Effect of pH on metal ions recoveries, by 5 ml nitric acid 4 M solution. The general conditions: modified Al2O3 NPs amount, 1 g; SDS amount, 50 mg, 3-mercapto- D-valin amount, 45 mg; Electric mixer-assisted extraction time, 15 min; metal ions concentration, 50 μg·L−1.
batch column method. In batch method, the shaking was continued up to 15 min (time all understudy metal ion was quantitatively by modified solid phase) and the uptake metal ion was eluted by 5 mL of 4.0 mol·L−1 HNO3. The sorption and desorption were repeated several times on same adsorbent and the eluted metal ion each time was evaluated. The capacity of this proposed sorbent did not change (with a normal variation of 2%) in 10 times repeat.
3.2.7. Effect of Interferences
In view of the fact that flame atomic absorption spectrometry provides high selectivity, the only interference may be attributed to the preconcentration step. The interfering effects of coexisting ions in ternary mixtures of Zn(II), Cu(II) and Fe(II) with potentially interfering ions were studied on the percent recovery of target ions. The tolerance limit was set as the amount of potentially interfering ions required to cause a ±4% error. To perform this study, various salts were added individually to a solution containing 0.1 μg·L−1 of Zinc, Iron and Copper ions, and the suggested procedure was applied. Table 1 shows the tolerance limits of the interference ions. The results demonstrate that the presence of large amounts of species commonly present in real samples, have no significant effect on the SPE of Zinc, Iron and Copper. It should be noted that individual investigations showed that the ions K+, Na+, Cl−, 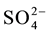 , Ca2+, Mg2+, Ba2+, Hg2+, Ni2+, Co2+ and Mn2+ ions were not adsorbed at pH 7.0 onto the sorbent up to concentrations given in Table 1. This shows selectivity of the sorbent for the analytes.
, Ca2+, Mg2+, Ba2+, Hg2+, Ni2+, Co2+ and Mn2+ ions were not adsorbed at pH 7.0 onto the sorbent up to concentrations given in Table 1. This shows selectivity of the sorbent for the analytes.
3.2.8. Analytical Features
The analytical features of the presented method such as linear range of the calibration curve, limit of detection and precision were also examined. By applying the optimum experimental conditions, the characteristics of the method are presented in Table 2. Along the limit of detection (LOD) of a method (the lowest analyte concentration that produces a response detectable above the noise level of the system), based on the three tames the standard deviation of the blank (N = 6) which was for all ions is presented in Table 2.
![]()
Table 1. Effects of the matrix ion on the recoveries of the examined metal ions.
aAverage of three measurements.
![]()
Table 2. Specification of presented method at optimum conditions for each element.
aDetermined as 3 SB/m (where SB and m are the standard deviation of the blank signal and the slope of the calibration graph, respectively).
3.2.9. Investigation of Preconcentration Factor
The elution volume strongly affects the preconcentration factor, defined as the ratio of sample volume to elution volume. By applying the optimum condition volumes up 500.0 mL of 50 µg·L−1 of Fe2+, Zn2+ and Cu2+ can be completely adsorbed on the column and could be easily desorbed (by 5.0 mL of HNO3 solution) and detected by FAAS. This shows that a preconcentration factor of 100.0 is achievable by the system.
3.2.10. Application to Environmental Samples
In order to demonstrate the applicability and reliability of the method for real world samples, four real samples, including tap water, wastewater, apple and mango samples were collected and analyzed by the method. The criterion of reliability such as accuracy and RSD was examined by standard addition method. The percentage of recoveries and RSD of each Zn2+, Fe2+ and Cu2+ ions in spiked food and water samples are given in Table 3 and Table 4. It was found that the Zn2+, Fe2+ and Cu2+ ions can be enriched and determined with high recoveries with RSD lower than 3%.
4. Conclusion
A simple, sensitive and selective method was developed for the preconcentration of Zinc, Iron and Copper in various real samples. In this study, a sorbent, 3-mercapto-D-valin-γ-Al2O3 (nanoparticles), was prepared by a simple and rapid modification method. As far as our information concern, this is the first time that the 3-mer- capto-D-valin have been used as a modifier material for the selective solid phase extraction of Zn(II), Fe(II) and Cu(II) from environmental and biological solutions prior to their determination by FAAS. The method has the following advantages: it is reproducible and has a high preconcentration factor and low analysis cost. These nanoparticles have relatively high adsorption as compared to the similar materials because of their smaller size. The reusability of the sorbent was greater than 10 cycles without any loss in its sorption behavior. All of the optimized parameters were employed in real spiked samples. The results showed that this method had a high potential for use in real samples with acceptable recovery and good precision. It shows that the suggested method allows the determination of sub μg·L−1 levels of Zinc, Iron and Copper. The method is economical due to the possibility of multiple uses of the sorbent. The method is simple, accurate and can be applied for the determination of analyte in environmental samples. The comparison of the results found in the present study and some recent studies on Zinc, Iron and Copper was given in Table 5. Most of the previously reported solid phase extraction methods in view of their advantage suffer from limitation such as low preconcentration factor. In our sense,
![]()
Table 3. Analytical results for the determination of (ΙΙ), Fe(ΙΙ) and Cu(ΙΙ) in apple and mango.
aAverage of four measurements.
![]()
Table 4. Analytical results for the determination of Zn(ΙΙ), Fe(ΙΙ) and Cu(ΙΙ) in tab water and waste water.
aAverage of three measurements; bWater was collected in a glass container from our research laboratory in Faculty of Chemistry, Islamic Azad University at Firouzabad city; cWastewater sample was collected from of Shiraz Industrial; dNo Detected.
![]()
Table 5. Comparative data from some recent studies for preconcentration of trace metals.
this limitation can be overcome by the highest sample volume (500 mL) to the lowest eluent volume (5.0 mL) and the preconcentration factor result was 100.’
Acknowledgements
We gratefully acknowledge the support of this work by the Shiraz Payame Noor University Research Council, Shiraz, Iran.
NOTES
*Corresponding author.