Which Yields with Wastes? Study on Pilot of the Efficiency of Water Treatment Plant— Case of the Sector of Algerian Tanning ()
1. Introduction
Tannery is an industry with strong potential of pollution. The incidences upon the environment which it is advisable to take into account understand not only the charges and the concentration of the traditional pollutants, but also the use of certain chemicals. Water is mixed with organic matter (proteins, peptides, and amino, acid ac- ids fatty, sulfur and trivalent chromium). They are highly polluted [1] . The majority of operations use and consume several chemical products. The animal skin is subjected to deferent process to eliminate meat, lipids and the hairs. This stage uses different chemicals products (in particular: hydroxide of sodium, hypochlorite of sodium, dichromate of potassium, lime, chlorides, sulphuric acid, formic acid, tensioactifs, sulphide of sodium, salts of sodium and ammonium, etc.). The obtained skin is then handled by Cr3+ or by the plant tannins, the mineral salts and the coloring agents to obtain leather [2] . The used products end in waste water with a clear contribution in polluting load. These operations are carried out in aqueous environment, therefore generate water pollution. The tannery waste water pollution has two sources [3] : Skin and Chemical reactive used in the various operations.
Without water purification (dysfunction of the installations) and in the absence of an effective technology of recovery, the unit loses annually on one hand:
・ 140 kilogram/day of chromium with their prices with 1.22 ?kg, are equivalent to 51879.77 ?year;
・ 162.4 kilogram/day of sulfides with their prices with 0.3 ?kg, are equivalent to 16577.76 ?year.
This heavy heritage of industry consuming water and import chemicals, the managers are forced to exert a responsibility. Management that implies, for example, to know well the chemicals implemented in the process (including the very prepared products). To take the safety measures for the protection of people, the environment and finally optimizes the operation of the waste water treatment installations in order to answer the multiple requirements and environmental stakes, social and economic [3] . Waste management and environmental protection are mandatory requirements of modern society. Nevertheless these treatments installations of polluting water represent an unbearable because of the high cost of operation (chemical, energy, maintenance).
On the other hand in Algeria, the steel industry activity’s generates an important production of industrial waste (slag) which raises a problem of storage and pollution. Following the example of industrial nations this product, presents a plentiful raw material or low cost. Who must be exploited on a large scale in operations “sometimes without interest for the administrators” for example the operations of waste water treatment [4] [5] .
The purpose of this research is to choose among the existing waste that can be reused. Within the framework of the application of the best available techniques, the goal is double: reusing waste and substitution with other products used in processes.
2. Material and Methods
2.1. Analysis of the Waste Water
2.1.1. Points of Taking
Several campaigns of data collecting were led to the exit of the stages of production, and at the level of the pond of collection where moved towards all the waste water generated by the company. Two modes data collecting are adopted:
Immediate grips and average samples constituted by the mixture of several collect distributed throughout the day at the rate of a collecting per hour.
2.1.2. Analyzed Parameters
Sulfur and other chemical parameters were analyzed according to the normalized (standardized) methods AFNOR [6] .
3. Experimental Study of the Effectiveness of the Station
To study the effectiveness of purification station of the unit by the new substituent, we carried out a partner of taking away of basic effluents (rejection between 6 A.M and 10 A.M of the morning) and acid (rejection between 10 A.M and 14 P.M), starting from the gutters of collection.
However, the objective of our partner was obtaining representative data collecting with an aim of being able to study on pilot the effectiveness of the station, and for this reason we based ourselves on the principal operations of purification, namely the desulfurization and precipitation of chromium.
3.1. Desulfurization
3.1.1. Principle
The elimination of sulfur contained in water of river, is carried out by oxidation with contribution of air according to reaction, refer to “(1)”
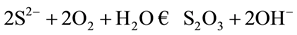 (1)
(1)
Thus the sulfur will be transformed into thiosulfate. The Pourbaix curves clearly show the distribution of the species of sulfur according to the pH and the potential (see Figure 1) [5] .
3.1.2. Procedure
To desulphurize the effluents of pre-hardening and hardening, a volume of effluent is collected at the end of the operations chain containing considerable quantities of sulfur. The rejections will be oxidized one taking account certain necessary parameters for the optimization of desulfurization results: (contribution of oxygen (O2), mechanical agitation, time):
・ Firstly: A contribution of air, during 3 hours using a compressor (a volume of anti-foamer is added to the reaction to avoid the foam formation which could obstruct the good diffusion of the air and thus the transfer of oxygen in water.
・ Secondly: Then by agitation during 3 hours, using a surface aerator (propeller electro-agitator);
・ Thirdly: Then a volume of manganese sulphate adds some MnSO4 (catalyst to be substituted there after by the ferrous waste of the iron and steel industry) used to obtain a complete and more rapid.
3.1.3. Experimental Results
Initial volume of effluent = 464 mg/l.
3.1.4. Interpretation of the Results
The test and the proportioning of sulfur by ferricyanides of potassium show us that the elimination of sulfur by oxidation to the air in the presence of catalyst MnSO4 gives excellent results. Elimination of 99.43% of sulfur (refer to Graphics 1-3). These results will be taken as means of comparison with the results of the substitute (ferrous waste resulting from the activity of iron and steel industry). The results obtained, show us that the treatment of desulphurization envisaged is very effective. However the reaction time and the operating conditions must be well controlled (parameters). The study on pilot of the influence of these parameters with use of MnSO4 like catalyst, gives us the results (see Table 1 and Table 2).
Indeed the results obtained, show us clearly and allow us to say that the best desulfurization is carried out by oxidation with contribution of air, and that simple agitation without contribution of air will not be enough to in any case with the desulfurization of the effluent ensures. But the frequent use of MnSO4 costs very expensive to the company (product of importation) and that always presents a charge leather for the industry.
![]()
Figure 1. Diagram of steady balances tension-PH of sulfur?water system, at 25˚C [5] .
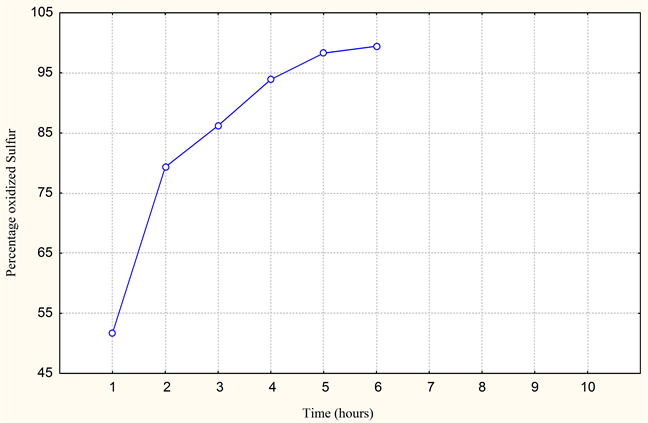
Graphic 1. Percentage of oxidized sulfur (with 2 ml MnSO4).
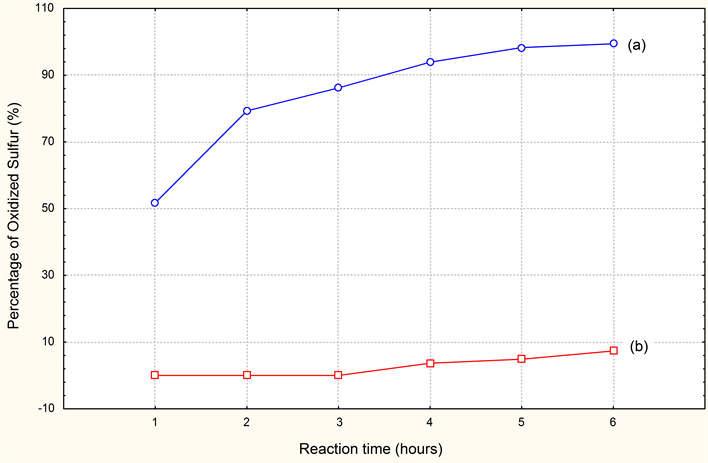
Graphic 2. Comparison of the desulfurization results with (2 ml of MnSO4). (a) With variation parameters (Contribution of air and agitation); (b) Without variation parameters (without contribution of air agitation).
3.1.5. Test of Substitution of the MnSO4 Catalyst
1) Objectives:
In order to decrease the importation of the products and thus to reduce the very high cost in currency for the leather company on the one hand, to find other exits for waste of another company of other share. We carried out tests of substitution of one of the products used in the station of waste water treatment (catalyst sulphate MnSO4 manganese) by another product (ferrous waste resulting from the iron and steel industry) available in Algeria and from which the chemical characteristics are not very different from that of catalyst used. The product used for substitution is FeSO4.
While being based on the fact that this product, when is oxide is become oxidants very strong and can thus take part in the oxidation of sulfur and replace catalyst envisaged MnSO4.
2) Experimental results of substitution:
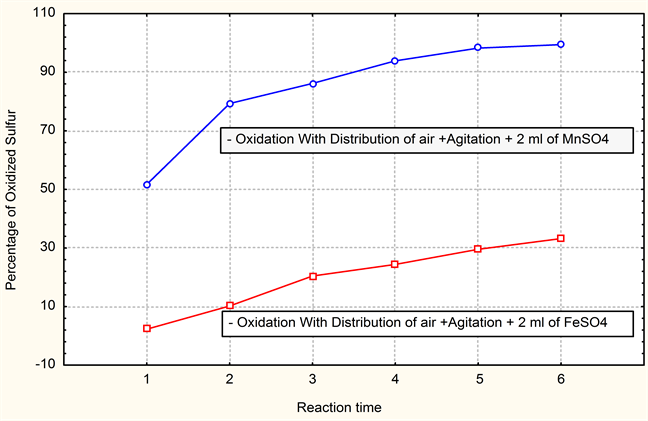
Graphic 3. Comparison of the results of percentage desulphurized sulfur.
![]()
Table 1. Percentage of oxidized sulfur (with 2 ml MnSO4).
![]()
Table 2. Comparison between the desulfurization results −2 ml MnSO4? without and with parameters variation.
For the oxidation of sulfur with use of the FeSO4 catalyst (Table 3 and Table 4).
・ Test: 10 ml;
・ Sulfur concentration: 2816 mg/l.
4. Discussion
From the tests on pilot, it arises that the MnSO4 catalyst used for the oxidation of sulfur definitely more effective and in conformity than the ferrous sulphate FeSO4 compared here to ferrous waste of the iron and steel sector.
![]()
Table 3. Desulfurization with distribution of air and low of FeSO4 = 0.41 ml/hour + agitation.
![]()
Table 4. Desulfurization without distribution of air and flow of FeSO4 = 2 ml/hour + agitation.
That could be the consequence proportion of iron which precipitates neighbors pH from 4.6 to 6 and thus does not take part has the oxidation of sulfur, whereas that of sulfur takes part has close pH has 8. The Pourbaix curves also inform us about this aspect of appearance of new species according to the pH [7] . We agree has to also say that better results can be obtained by increasing the concentration of the substituent (ferrous waste) in the effluent of the tannery and by also increasing the reaction time of desulfurization. The solution proposed could be used as springboard for possible reflection for the complex problems of companies which note that their sustainability is threatened because of the loads which can be to relieve by rational solutions. The companies has great possibilities of preserving the natural environment and of minimizing it the economic losses, by, on the one hand, the application of the concept of substitution and or recycling of waste valuable and on the other hand by a management responsible for all under systems for the company (station of purification for example). Finally, at least as far as general Environmental management systems, is concerned, the environmental policy of an enterprise must include a commitment to preventing pollution. In the United States, the common term for the body of knowledge, approaches, techniques, practices, and technologies aimed at minimizing the creation of pollution is “pollution prevention”, or “P2”. Terms often used as near synonyms for P2 are “waste minimization” and “clean production”. Together these terms sum [8] .
5. Conclusion
Stricter legal limits on sulfur levels require measures to reduce their value in the wastewater final tannery. Removal of wastewater containing high sulfur concentrations contributes significantly to the overall effluent. However, the final sulfate concentration in the tannery wastewater is still too high to meet future standards. Separate results remain low, 32% for waste from the tannery, and 30% for the waste from the steel industry. But the sum of the results of the two waste give better results desulfurization, or 60% of oxidized sulfur. The companies have great possibilities of minimizing the economic losses, and to preserve the natural environment. The review of EU waste legislation offers the chance to revolutionize the way we think about and deal with waste. Turning waste into a valuable resource should be among the cornerstones of a resource-efficient in world. However, this will require combining ambitious targets with intelligent policy tools and rigorous application. Tannery industry shows a clear example of what can be gained if we succeed. Substitution by waste also underscores the enormous potential of waste to make a positive contribution to the environment and society as a whole.
Acknowledgements
The authors would like to thank Kamel Eddine BOUHIDEL and Hamel Benmoussa for their support and contribution to the work.