Improving Flow Property of Nifedipine Loaded Solid-Lipid Nanoparticles by Means of Silica for Oral Solid Dosage Form ()
1. Introduction
Oral drug delivery is the most convenient and preferred among all routes of drug administration. Of this oral delivery system, solid formulations, especially tablet, is the most acceptable, patient friendly and robust dosage form considering its favorable physico-pharmaceutical characteristics, low economic cost, simplicity in administration, and easiest transportation. When this dosage unit containing poorly water-soluble drugs is administered orally, insufficient dissolution and corresponding inadequate bioavailability might occur throughout the gastro-intestinal tract [1] . Developing strategies to improve drug dissolution rate and to enable the effective oral delivery for poorly water-soluble drugs is currently one of the greatest challenges to formulator in the pharmaceutical research field [2] [3] . Various approaches have been employed to tackle this limitation [4] - [9] and nano-based drug delivery systems have greater potential. This system have been applied for a variety of areas, such as multiple targeting, in vivo imaging, extended circulation time, and systemic control release, and it can be successfully used as drug delivery tools for currently available bioactive compounds [10] . Recently, lipid-based nanoparticulate systems such as microemulsions, solid-lipid nanoparticles (SLNs), and nanoemulsions have been proved as one of the useful approaches to improve solubility and absorption of drug [6] .
SLNs, which are composed of the matrix of biocompatible solid lipids like triglycerides, fatty alcohols, phospholipids and waxes, have some advantages with respect to stability, drug release profile, and biocompatibility. Few limitations of SLN dispersions, however, include physical instability arising due to particle aggregation and gelation under accelerated storage conditions [11] . This drawback can be overcome by conversion from its dispersion to dry powder. Lyophilization has been the most common method to convert SLN dispersion to dry form [12] . Recently, we developed a newly formulated solid-lipid nanoparticle of nifedipine (NI-SLN) using only two solid phospholipids, e.g., hydrogenated soybean phosphatidylcholine (HSPC) and dipalmitoylphosphatidylglycerol (DPPG), without any emulsifier and organic solvent in the form of aqueous dispersion by high pressure homogenization technique. Although NI-SLN dispersions were stable for up to 4 months at 6˚C, a specialized storage condition [13] , lyophilization technique with trehalose (a disaccharide) as a cryoprotectant enhanced stability for up to 6 months at 30˚C and 65% RH [14] . Moreover, no deterioration of the oral bioavailability of the encapsulated drug, along with hemocompatiblity was observed [15] [16] . However, considering conclusive oral formulation using this SLN, development of SLN powder with an adequate powder flow property is necessary because only powder of SLN with trehalose is easily expected to show low flowability.
Since 2001, silica-based nanoparticles have been recognized as a promising drug delivery system using porous silica, in particular, mesoporous material MCM-41 was firstly proposed [17] . Porous silica possesses several attractive features including high surface area and pore volume, good biocompatibility, chemical inertness as well as being able to modify the surface and pore size, affording control over the drug release [18] - [23] . The enhanced dissolution rate of drugs is based on the adsorption of drug molecules by Van der Waals forces and hydrogen bonds to the surface and pores of the silica carrier. The bonds can be easily broken when in contact with water and the drug molecules are consequently released. It is worth mentioning that despite the significant progress in the utilization of porous silica for drug delivery applications, there are only a few studies that demonstrate the silica formulations for lipid emulsions. For example, a versatile silica composite fabricated with lipid emulsion of celecoxib, a non-steroidal anti-inflammatory drug, have been reported to show its free flowing property satisfactorily after lyophilization, which might be employed to formulate an oral solid dosage form [24] . In another study, silica-lipid microparticles were reported to attain better control and predictability over drug release, solubilization, and in vivo pharmacokinetics [25] [26] . Furthermore, a surfactant free solid-lipid microparticle prepared by hot melt process and coated with various types of silica was also reported to modulate the kinetics of ibuprofen release [27] . Nevertheless, there have been no reports to date of a surfactant-free SLNs obtained by high pressure homogenization technique, of which the release behavior can be influenced in presence of porous silica, ultimately increasing the flow property of powdered nanoparticles. We assume that, as the physical state of lipid component in NI-SLNs is solid, unlike the lipid emulsion, the adsorption characteristics of SLNs on the surface of silica may differ from that of emulsion type NPs attributed to the different mechanisms in the formation of strong/weak chemical bonding. Therefore, a study of the functionality of porous silica on the drug release behavior along with its physicochemical characteristics of SLNs presents a major interest to promote the applications in formulating an oral solid dosage form.
In the present study, two types of hydrophilic silica named Aerosil® and Carplex® were employed to prepare silica nanocomposite (SNC) for NI-SLN because of their different characteristics such as particle size and pore size. NI-SLN was prepared by high pressure homogenization technique using two phospholipids, HSPC and DPPG, and lyophilized with different types of silica and trehalose, which was used as a cryprotectant [14] . The physicochemical, morphological, pharmaceutical, in vitro drug release characteristics were also investigated.
2. Materials and Methods
2.1. Materials
Hydrogenated soybean phosphatidylcholine [COATSOME®NC-21(HSPC)] and dipalmitoylphosphatidylglycerol [COATSOME® MGLS-6060 (DPPG)] were purchased from Nippon Oil and Fats Co., Ltd. (Tokyo, Japan). Nifedipine (JPXIV, NI) was provided by Nippon Fine Chemical Co., Ltd. (Osaka, Japan). Trehalose was purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). Silica (Aerosil® and Carplex®) were kindly provided from Evonik Japan Industries Ltd. (Tokyo, Japan). The membrane filters (pore size: 0.20 and 0.45 µm) were purchased from Toyo Roshi Kaisha Ltd. (Tokyo, Japan). All reagents were of the highest grade commercially available and all solutions were prepared using de-ionized distilled water.
2.2. Experimental
2.2.1. Preparation of Nifedipine (NI)-loaded Solid-Lipid Nanoparticle Dispersion
To prepare the NI-loaded solid-lipid nanoparticle dispersion (SLND), 40 mg of NI and 1000 mg of lipids (HSPC: DPPG, 5:1 molar ratio) were added to a mortar and physically mixed for 5 min. The mixture was then co-ground by a roll mill (R3-1R, Kodaira Seisakusho Co., Ltd., Tokyo, Japan). The co-grinding cycle was repeated 10 times. The resultant roll mixture was dispersed in 200 ml of de-ionized distilled water and premixed using a Speed Stabilizer (10,000 rpm, Kinematica Co., Luzern, Switzerland) at 9000 rpm for 10 min, followed by high pressure homogenization (max pressure: 200 MPa, Microfluidizer®, M110-E/H; Microfluidics, Co., Newton, MA, USA) with a pass cycle of 100 [28] . The suspension thus obtained was filtered through a 0.2 µm membrane filter and stored in a refrigerator at 6˚C until further use.
2.2.2. Lyophilization
To prepare lyophilized product, the SLND was collected into small vials followed by the addition of 2.5% w/v aqueous solution of trehalose (TS) at a ratio 1:1 (SLND: TS) and mixed well. Each vial was then frozen at −40˚C for 3 h and the frozen sample was freeze-dried in a glass chamber for 24 h using a vacuum pump accompanied by a vapor condenser (−20˚C, 0.0225 Torr). Then, secondary drying was carried out at 20˚C for 24 h. This lyophilized product was simply labeled as SLN and used as control.
2.2.3. Preparation of Lyophilized Silica Nanocomposites (SNC) of Nifedipine Solid-Lipid Nanoparticle
For the preparation of SNC, silica was well dispersed in water first by means of a magnetic stirrer in a beaker for 30 min. Again, both SLND and the silica dispersion (using silica-to-lipid ratio = 1:9 and 2:8) were agitated using a magnetic stirrer in a beaker for 30 min to allow the adsorption of nanoparticles by silica. This mixture (silica nanocomposite dispersion) equivalent to 2 ml of SLND was transferred to small vials. TS at a concentration 2.5% w/v was added at a ratio 1:1 (SLND: TS) and mixed well. Each vial was then frozen at −40˚C for 3 h and the frozen sample was freeze-dried in a glass chamber for 24 h using a vacuum pump accompanied by a vapor condenser (−20˚C, 0.0225 Torr). Then, secondary drying was carried out at 20˚C for 24 h. These lyophilized SNC were labeled according to the first alphabet of the name of silica used and its concentration. For example, SLN-A200 (10%) and SLN-C67 (20%) are attributed to nanocomposite containing NI-SLNs with Aerosil-200 at silica-to-lipid ratio, 1:9, and with Carplex-67 at silica-to-lipid ratio, 2:8, respectively. The labels and compositions of the different SNCs used in this work are described in Table 1.
2.3. Particle Characterization
2.3.1. Carr’s flow ability index and Hausner ratio
To measure the flow property, SNCs with a silica-to-lipid ratio of 2:8 were prepared as described in section 2.2.3. Flow ability of the powders were determined by following Carr’s method [29] . Briefly, a measured quantity (about 0.5 g) of powder passed through 1 mm sieve was taken in a 5 cc (0.1 cc graduated) measuring cy-
![]()
Table 1. Composition of silica nanocomposites.
linder of about 10 mm in diameter and the unsettled volume was recorded. The cylinder containing powders was then tapped 250 times [30] manually and the volume was recorded again. The Carr’s index (CI) and Hausner ratio (HR) was calculated using the following equation:
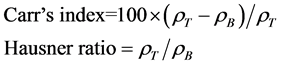
where, ρT and ρB represent the tapped density and bulk density, respectively. Both the tests were repeated 3 times.
USP categorize powders according to the scale of flowability depending on the CI and HR value as, ≤10 and 1.00 - 1.11: Excellent; 11 - 15 and 1.12 - 1.18: Good; 16 - 20 and 1.19 - 1.25: Fair; 21 - 25 and 1.26 - 1.34: Passable; 26 - 31 and 1.35 - 1.45: Poor; 32 - 37 and 1.46 - 1.59: Very poor; and >38 and >1.60: Very, very poor, respectively.
2.3.2. Scanning electron micrographs (SEM)
Scanning electron micrographs (SEM) of the SNCs were obtained using a scanning electron microscope (SSX- 500, Shimadzu, Tokyo, Japan) after platinum metallization. An accelerating voltage of 15 kV was used.
2.3.3. Mean particle size (PS), polydispersibility index (PDI), and zeta potential (ZP)
De-ionized distilled water (2 ml) filtered through a membrane filter (0.20 µm) was added to the vial containing the freeze-dried samples, and the vial was shaken by vortex agitation to rehydrate them. The PS and PDI were measured by dynamic light scattering (Nano ZS, Malvern Instruments, Worcestershire, UK), and ZP was estimated on the basis of electrophoretic mobility under an electric field at room temperature. The PS was analyzed based on weight distribution.
2.3.4. Recovered potency
The freeze dried samples were re-constituted as described in section 2.3.3. Exactly 0.2 ml of reconstituted sample was taken in an eppendorf tube (1.5 ml) and 0.8 ml of methanol was added followed by sonication for 3 min. It was then centrifuged at 7000 rpm for 10 min. The supernatant was filtered through a 0.2 µm syringe filter. The drug content was assayed by RP-HPLC (Shimadzu, Tokyo, Japan) in a binary mode (dual pump), with a UV detector at a wavelength of 236 nm. The HPLC column was reversed phase (Cadenza 5CD-C18, 4 mm ID × 150 mm; Imtakt) at 40˚C. The mobile phase of methanol: aqueous formic acid (0.2% v/v) at a ratio of 60:40 was delivered at a flow rate of 0.4 ml/min. The retention time of NI was found to be 6.1 ± 0.1 min. A calibration curve with at least three standard concentrations (10, 50, and 100 µg/ml) of NI was used.
2.3.5. Aqueous re-dispersibility study
Aqueous re-dispersibility study of the lyophilized samples was performed by mixing both freeze-dried SLN (control) and SNCs equivalent to 190 µg of NI in 10 ml of water contained in a 20 ml beaker, stirred continuously at 100 rpm at room temperature. The analytic samples (0.3 ml each) were withdrawn at intervals of 1, 5, 10, 20, 30, 60, 90, and 120 min, followed by the replacement of an equal volume of water. The solution was then filtered through a membrane filter (0.45 µm) to remove any aggregates of silica nanocomposites. The amount of NI dispersed in the solution was monitored using the HPLC method as described in Section 2.3.4 and the ratio of NI released (%) was calculated.
2.4. Statistics
Statistical analyses were performed using the Student’s t-test with the probability value, p < 0.05 indicating significant difference.
3. Result and Discussion
In this study, silica nanocomposites were prepared using two hydrophilic fumed silica, Aerosil®200, and Aerosil®380 and three hydrophilic precipitated silica, Carplex®67, Carplex®80, and Carplex®FPS 500. Among them Aerosil®200 was a nonporous silica. Table 2 represents the powder properties of the silica itself including mean particle size, specific surface area, and tapped density according to the certificate of analysis supplied by the manufacturing origin. In addition, the ZP values of aqueous dispersion (0.125% w/v) of each type of silica are also presented in Table 2. Figure 1 illustrates the differences in molecular properties between fumed and precipitated silica; precipitated silica (Carplex®) (Figure 1(a)) has a larger number of H-bond acceptors than that of fumed silica (Aerosil®) (Figure 1(b)). Therefore, the opportunity for the formation of any chemical bonding between silica and SLN is considered to be greater by precipitated silica.
Figure 2(a) and Figure 2(b) show the values of Carr’s Index (CI) and Hausner ratio (HR) of the SNCs containing 20% of SLN, respectively. The CI value of SLN (control) showed 26.5; the smallest value of CI (12.78) was attributed to SLN-C80; and the highest value (37.47) was from SLN-A200. In addition, Figure 2(b) showed the HR value of SLN-C80, as lowest (1.14) and SLN-A200 as highest (1.59). Therefore, the use of silica in the preparation of the nanocomposite improved the overall powder flow characteristics significantly to reach-
![]()
Table 2. Powder and electrostatic properties of silica.
aValues taken from the certificates of analysis supplied by the manufacturer of silica; bValues were obtained using the method described in section 2.3.3 using 0.125% w/v dispersion of silica.
![]()
![]() (a) (b)
(a) (b)
Figure 1. Chemical structure of silica, (a) Aerosil® and (b) Carplex®, highlighting the diffe- rence in hydrogen bond acceptors.
![]()
![]() (a) (b)
(a) (b)
Figure 2. Powder flow property of freeze dried silica nanocomposites (a) Carr’s compressi- bility index, and (b) Hausner ratio. SLN: Nifedipine solid lipid nanoparticle (NI-SLN) lyophi- lized with 2.5% w/w trehalose; SLN-A200: SLN with Aerosil-200 (20%); SLN-A380: SLN with Aerosil-380 (20%); SLN-C67: SLN with Carplex-67 (20%); SLN-C80: SLN with Car- plex-80 (20%); SLN-FPS: SLN with Carplex FPS-500 (20%). Each value represents the mean ± SD (n = 3); *p < 0.05, **p < 0.01 versus each value of SLN (control).
from the “passable” to “good” categories when compared with lyophilized SLN, with the exception of Aerosil®200. This may be due to the non-porous nature of this silica leading to the inadequate adsorption of SLNs on its surface. Also, as another reason, tapped density of silica may be involved in this effect; namely, all nanocmposites with Aerosil®, having smaller tapped density value, showed the higher CI and HR values than that with Carplex®, having larger tapped density value. Among these SNCs, SLN-C80, SLN-F500, and SLN-C67 indicated “good” and “fair” flowability, respectively. The physical properties shown in Table 2 implied that the tapped density of silica as a vehicle might be critically important for the flowability of its nanocomposites. But, the reason of fair flowability of nanocomposites with Carplex®FPS500 although having higher tapped density might be due to poor adsorption characteristics of SLN by silica.
The solid-state surface morphology in the SEM images (Figure 3) showed a three-dimensional internal network of SNCs forming aggregates. Among the Aerosil® nanocomposites, the surface of SLN-A200 was more uneven than SLN-A380. This aligns with the HR and CI values obtained for SLN-A200, which had less flowability than SLN-A380 (Figure 2). On the other hand the regular spherical shaped microparticles with smooth surface were observed by Carplex® nanocomposites, which can be defined as powders with more flowability than the Aerosil® nanocomposites by using CI and HR values. Notably, of the Carplex® nanocomposites, more smooth surfaces of particles seemed to be present in SLN-C80. As the sphericity and surface smoothness of a particle are essential criteria of a powder to be flowable, this SEM results further proved that precipitated silica has the efficiency to increase the flow property of a nanocomposite entity.
The inclusion of silica has the potential to significantly affect the physicochemical parameters of the SLNs, such as PS, PDI, ZP, and recovered concentration due to possible interactions between SLNs and silica. Notably, these parameters are indicators for the long storage stability of nanocomposites. As shown in Figure 4, the PS (nm) of the SNCs was found comparatively smaller with higher silica-to-lipid ratio (2:8) and vice versa, with the exception of the nanocomposite formed by Carplex FPS®500. Especially, among the SNCs, SLN-A200 (20%), SLN-C80 (20%), SLN-FPS (10%), and SLN-C67 (20%) exhibited PS below 100 nm. In addition, the PDI values in Figure 5 stated more uniform particle distribution (PDI < 0.3) in SLN-C67 (10%) and SLN-A380 (20%). However, although these values of nanocomposites were slightly higher than that of SLN, this might be owing to the presence of released SLN from the composites. Given that released SLN and silica co-existed in the solutions, wider particle size distributions might be observed.
ZP is responsible for the aggregation of particles in storage. Previously, electrostatic stabilization of SLNs was reported to require a ZP ≥ 30 mV or ≤−30 mV. It has also been reported that a ZP between 5 and 15 mV results in limited flocculation and a ZP between −5 and −15 mV leads to maximum flocculation [31] . The SLN itself showed a ZP around −50 mV (Figure 6). The silica entities, themselves, used to prepare nanocomposites also showed their ZP values around −25 mV by Aerosil®, and −30 mV by Carplex® (Table 2). Nevertheless, no silica component played an unfavorable role for noticeable change in ZP of SNCs. Almost all SNCs showed
![]()
Figure 3. SEM images. SLN: Nifedipine solid lipid nanoparticle (NI-SLN) lyophilized with 2.5% w/w trehalose; A200: Aerosil-200; A380: Aerosil-380; C67: Carplex-67; C80: Carplex- 80; CFPS: Carplex-FPS 500; SLN-A200 (10%) or (20%): SLN with Aerosil-200 (10% or 20%); SLN-A380 (10%) or (20%): SLN with Aerosil-380 (10% or 20%); SLN-C67 (10%) or (20%): SLN with Carplex-67 (10% or 20%); SLN-C80 (10%) or (20%): SLN with Carplex-80 (10% or 20%); SLN-FPS (10%) or (20%): SLN with Carplex FPS-500 (10% or 20%), respec- tively. All silica nanocomposite samples were lyophilized with 2.5% w/v trehalose and freeze dried.
![]()
Figure 4. Mean particle size of silica nanocomposites. SLN: Nifedipine solid lipid nanopar- ticle (NI-SLN) lyophilized with 2.5% w/w trehalose (as control); SLN-A200 (10%) or (20%): SLN with Aerosil-200 (10% or 20%); SLN-A380 (10%) or (20%): SLN with Aerosil-380 (10% or 20%); SLN-C67 (10%) or (20%): SLN with Carplex-67 (10% or 20%); SLN-C80 (10%) or (20%): SLN with Carplex-80 (10% or 20%); SLN-FPS (10%) or (20%): SLN with Carplex FPS-500 (10% or 20%), respectively. The control and all silica nanocomposite samples were lyophilized with 2.5% w/v trehalose and freeze dried. Each value represents the mean ± SD, (n = 3); *p < 0.05, **p < 0.01 versus each value of SLN (control).
![]()
Figure 5. Polydispersity index (PDI) of silica nanocomposites. SLN: Nifedipine solid lipid nanoparticle (NI-SLN) lyophilized with 2.5% w/w trehalose (as control); SLN-A200 (10%) or (20%): SLN with Aerosil-200 (10% or 20%); SLN-A380 (10%) or (20%): SLN with Aerosil- 380 (10% or 20%); SLN-C67 (10%) or (20%): SLN with Carplex-67 (10% or 20%); SLN-C80 (10%) or (20%): SLN with Carplex-80 (10% or 20%); SLN-FPS (10%) or (20%): SLN with Carplex FPS-500 (10% or 20%), respectively. The control and all silica nanocomposite samples were lyophilized with 2.5% w/v trehalose and freeze dried. Each value represents the mean ± SD (n = 3); *p < 0.05, **p < 0.01 versus each value of SLN (control).
![]()
Figure 6. Zeta potential (ZP) of silica nanocomposites. SLN: Nifedipine solid lipid nanopar- ticle (NI-SLN) lyophilized with 2.5% w/w trehalose (as control); SLN-A200 (10%) or (20%): SLN with Aerosil-200 (10% or 20%); SLN-A380 (10%) or (20%): SLN with Aerosil-380 (10% or 20%); SLN-C67 (10%) or (20%): SLN with Carplex-67 (10% or 20%); SLN-C80 (10%) or (20%): SLN with Carplex-80 (10% or 20%); SLN-FPS (10%) or (20%): SLN with Carplex FPS-500 (10% or 20%), respectively. The control and all silica nanocomposite samples were lyophilized with 2.5% w/v trehalose and freeze dried. Each value represents the mean ± SD (n = 3); *p < 0.05, **p < 0.01 versus each value of SLN (control).
their ZP values from −30 to −50 mV indicating high stability, with the exception of SLN-A200 (20%).
It is important that the recovered potency of a drug is responsible for the desired level of drug release. All the SNC samples showed high drug recovery, i.e., above 80% (Figure 7). It might be easy to understand that different silica might have different degree of bonding capacity with solid-lipid nanoparticles resulting in variation of drug recovery. In addition, the re-dispersibility study of SLN (control) and SNCs were also investigated under ambient condition using water as a medium (Figure 8). All samples showed rapid dissolution ratios and the values remained constant, indicating that around 60% NI-SLNs were easily and rapidly released from SNCs.
![]()
Figure 7. Recovered potency (%) of silica nanocomposites. SLN: Nifedipine solid lipid nano- particle (NI-SLN) lyophilized with 2.5% w/w trehalose (as control); SLN-A200 (10%) or (20%): SLN with Aerosil-200 (10% or 20%); SLN-A380 (10%) or (20%): SLN with Aerosil- 380 (10% or 20%); SLN-C67 (10%) or (20%): SLN with Carplex-67 (10% or 20%); SLN-C80 (10%) or (20%): SLN with Carplex-80 (10% or 20%); SLN-FPS (10%) or (20%): SLN with Carplex FPS-500 (10% or 20%), respectively. The control and all silica nanocomposite sam- ples were lyophilized with 2.5% w/v trehalose and freeze dried. Each value represents the mean ± SD (n = 3); *p < 0.05, **p < 0.01 versus each value of SLN (control).
![]()
Figure 8. Re-dispersibility profile of silica nanocomposites. SLN: Nifedipine solid lipid nanoparticle (NI-SLN) lyophilized with 2.5% w/w trehalose (as control); SLN-A200 (10%) or (20%): SLN with Aerosil-200 (10% or 20%); SLN-A380 (10%) or (20%): SLN with Aerosil- 380 (10% or 20%); SLN-C67 (10%) or (20%): SLN with Carplex-67 (10% or 20%); SLN-C80 (10%) or (20%): SLN with Carplex-80 (10% or 20%); SLN-FPS (10%) or (20%): SLN with Carplex FPS-500 (10% or 20%), respectively. The control and all silica nanocomposite samples were lyophilized with 2.5% w/v trehalose and freeze dried. Each value represents the mean ± SD (n = 3).
Previously, we reported that NI-SLN showed an increase in the 4-fold Cmax compared with NI pure drug; that is to say, enhanced drug absorption may have been observed in SNCs because SLNs were released to be intact form.
4. Conclusion
We successfully improved the flow property of powdered nanoparticles by formulating nanocomposite with various types of silica. The precipitated silica, Carplex®, and especially Carplex®80 was found to produce the most favorable results, i.e., with the greatest improvement in flowability and lack of deterioration during drug release. Therefore, this study suggests that Carplex® could be a novel excipient for use in pharmaceutical research and in the development of oral solid dosage form. Further work investigating the long-term stability of this new formulation is essential in the development of an established dosage form. Also a clinical trial would be essentially required to investigate how this new formulation shows the pharmacodynamics.
Acknowledgements
This investigation was possible owing to a research grant from the Japan Society for the Promotion of Science (JSPS). Therefore the authors would like to cordially acknowledge the JSPS for arranging such a grant through the RONPAKU program. Also, the authors thank Evonik Japan industries Ltd. for kindly providing some types of silica used in this study.
NOTES
*Corresponding author.