
Where (a) is the tetrahydrofuran molecule (C4H8O), (b) is the deoxyribose ring.
Several papers on electron interaction with either gaseous or surface deposited THF have been recently published, which consider electron spectroscopy of resonance-enhanced vibration excitation [2], electron-stimulated desorption yields of H− from thin films [3,4] and electron induced damage of solid THF film [5]. The earlier work on THF has also been summarized [5].
Since the discovery of the DNA double helix in 1953 [6], the structure of tetrahydrofuran is a kind of scientific controversy, for there are various descriptions. One of equilibrium geometries of THF is a twisted C2 group, which may be a transition structure [7,8]. However, ab initio calculation predicts that the equilibrium structure of THF is an envelope conformation of Cs [9], in which 4 of carbon atoms in a plane and oxygen atom out of this plane. Many of the works, specially, pay attention the structure of liquid tetrahydrofuran. Hydrogen/deuterium isotopic substitution neutron diffraction techniques have been used to measure the structural correlation functions of liquid tetrahydrofuran at room temperature [10]. The molecular interactions of pyrazine with THF are the weak Hydrogen bond N-C-H…O [11]. The tetrahydrofuran clathrate hydrate rapidly densifies at 77 K and ∼1.3 GPa. The densified sample is then heated to 150 K at 1.5 Gpa, and recovered at 1 bar and 77 K. Its X-ray and Raman measurements show that the densified sample is amorphous. The molecular arrangement of water in this sample appears to be similar to that in high-density amorphous ice [12].
2. DENSITY FUNCTIONAL THEORY
In DFT, electronic energy E is divided into several components,
 , (1)
, (1)
where ET is the electronic kinetic energy, EV is the potential energy including the electron-nucleus attraction and nucleus-nucleus repulsion, EJ is the electron-electron repulsion, and EXC is the exchange-correlation. Except nucleus-nucleus repulsion, each term can be expressed as the function of electron density 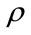
 , (2)
, (2)
 ,(3)
,(3)
 , (4)
, (4)
where all the three terms are the functions of electron density. EX(ρ) and EC(ρ) are the exchange and correlation functional, respectively, the former is a local functional only related with the electron density, and the later is a gradient-corrected functional related with the electron density and its gradient Ñρ. In 1988, Becke [13] derived the local exchange functional as follows:
 , (5)
, (5)
 , (6)
, (6)
where ρ is a function of r, 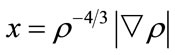 , γ is a parameter fitted with the exchange energy of noble atoms (γ = 0.0042 a.u.). Similarly, in 1992, Perdew and Wang [14]
, γ is a parameter fitted with the exchange energy of noble atoms (γ = 0.0042 a.u.). Similarly, in 1992, Perdew and Wang [14]
suggested a corrected functional as
 , (7)
, (7)
where

Here rs is the density parameter and ζ is the correlated spin polarization. DFT is a joint calculation with the exchange and correlation functional. The B3LYP method is to combine Becke’s exchange functional and the correlated functional of Lee, Yang and Parr, with both are gradient-corrected. The local correlation functional is from Vosko, Wilk and Nusair(VWN). Then the so-called Becke’s three parameter functional [15] is
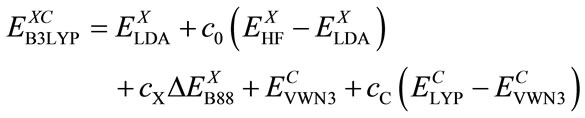 (8)
(8)
Using G1 method, Becke has obtained: c0 = 0.20, cX = 0.72 and cC = 0.81 by optimizing of exchange and correlation energy. The Eq.9 with the lower energy calculated will be used for the presented DFT-SCF calculations.
 (9)
(9)
3. THE THEORETICAL DERIVATION OF MOLECULAR ELECTRONIC STATE
The molecular electronic state is the irreducible representation of molecular group, therefore, before the calculation, it is necessary to derive the possible electronic states based on the resolution, reduction and product of group representation [16-19]. Now, we derive the possible electronic states for some of possible symmetry groups, for example, tetrahydrofuran (THF) will be possible group C2v, Cs, or C1.
For simplicity, we firstly derive the electronic state of furan (C4H4O), its ground state is known as  of C2v. It is supposed that we have the following reaction
of C2v. It is supposed that we have the following reaction
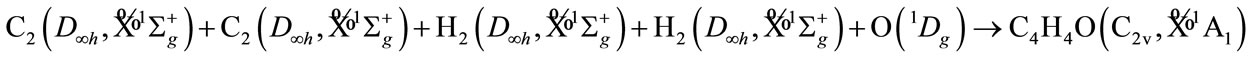
All the irreducible representation for given the reactive molecules with given state are resolved into the irreducible representation of molecular group C2v of C4H4O, as follows. Oxygen O atom must be the singlet excited state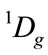 , in order to result the singlet ground state
, in order to result the singlet ground state  of furan based on the quantization vector addition of spin. The resolution of the irreducible representation, i.e. the electronic state for 2 of C2 and 2 of H2, all are the same as
of furan based on the quantization vector addition of spin. The resolution of the irreducible representation, i.e. the electronic state for 2 of C2 and 2 of H2, all are the same as
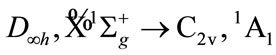
And the resolution of  of O atom is
of O atom is

The direct product will be

There will be component  for furan (C4H4O) i.e. the allowed electronic state.
for furan (C4H4O) i.e. the allowed electronic state.
For tetrahydrofuran (THF), we have the following reaction

Here, we only list these results by a table without the details of derivation.
The molecular property without indicating the symmetry and electronic state is meaningless. The electronic states derived here are proved by the following calculations.
4. CALCULATION AND COMPARISON
The present calculation is based on the density functional theory (DFT) described above and used the B3P86 method of Eq.9 with basis set 6-311G**.The calculated energy with B3P86 is usually the lowest one. The GAUSSIAN03 [20] package of program were employed to carry out all the calculations reported in this work.
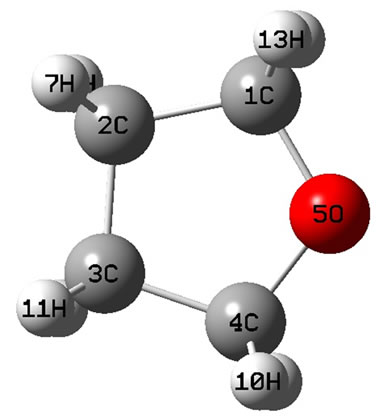
The calculated geometry of C2v is shown in Figure 1, where, 4 of carbon atoms C and oxygen atom O constitute a plane, i.e. 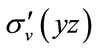 of C2v and C2 is z-axis passing through oxygen atom O and bisecting the opposite bond C-C. The electronic state is
of C2v and C2 is z-axis passing through oxygen atom O and bisecting the opposite bond C-C. The electronic state is  and triplet state
and triplet state 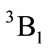 is in a high level by 6.569 eV than
is in a high level by 6.569 eV than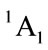 . Each carbon atoms C is not to be sp2 hybridization, for each carbon atoms C has four bonds to connect four of atoms, then, it will be impossible to have plane structure. In addition, the lowest two of 33 harmonic frequencies (cm–1) are negative, i.e. B1 = –222.7; A2 = –207.4 (To see the end of Table 1), this is an implicit assent of that C2v geometry would be a transition state, which is agreement with reference [7-9]. This is why to have energy level sequence C1 < Cs < C2v.
. Each carbon atoms C is not to be sp2 hybridization, for each carbon atoms C has four bonds to connect four of atoms, then, it will be impossible to have plane structure. In addition, the lowest two of 33 harmonic frequencies (cm–1) are negative, i.e. B1 = –222.7; A2 = –207.4 (To see the end of Table 1), this is an implicit assent of that C2v geometry would be a transition state, which is agreement with reference [7-9]. This is why to have energy level sequence C1 < Cs < C2v.
The calculated geometry of Cs is shown in Figure 2, where, 4 of carbon atoms C and oxygen atom O are not on a plane, atom O out of this plane. This is similar to the envelope Cs conformation [3]. The energy of Cs is a little lower than that of C2v. The lowest two of 33 harmonic frequencies (cm–1) are also negative, i.e. A" = –222.8; A" = –207.7 (To see the end of Table 2). Therefore, Cs would be a transition state, and is not a ground state which is contrary to that of [3].
The calculated geometry of C1 is shown in Figure 3 and Table 3. The energy of this state is lower by 0.16705 ev or 1347.3 cm−1 than that of C2v with . The harmonic frequencies (cm−1) for the C1 geometry are A = 53.91, A = 266.1, A = 576.1,···, A = 3080, A = 3113.8 and A = 3122.3. Moreover, the important thing is no negative value only for this conformation, Therefore, the ground electronic state is
. The harmonic frequencies (cm−1) for the C1 geometry are A = 53.91, A = 266.1, A = 576.1,···, A = 3080, A = 3113.8 and A = 3122.3. Moreover, the important thing is no negative value only for this conformation, Therefore, the ground electronic state is  for tetrahydrofuran (THF) in which 4 of carbon atoms C and oxygen atom O do not constitute a plane, but, like a “wave plane”, or the “helix surface” as shown in Figure 3(c), where the hydrogen atoms have been neglected.
for tetrahydrofuran (THF) in which 4 of carbon atoms C and oxygen atom O do not constitute a plane, but, like a “wave plane”, or the “helix surface” as shown in Figure 3(c), where the hydrogen atoms have been neglected.
5. CONCLUSION
The calculations with method B3P86/6-311G** show that the energy level sequence is 
 for tetrahydrofuran (THF). Although the symmetry of C2v is relatively high, the energy of
for tetrahydrofuran (THF). Although the symmetry of C2v is relatively high, the energy of  is not the lowest, or the highest one. And there are two of negative harmonic frequencies. Therefore, the C2v conformation with state
is not the lowest, or the highest one. And there are two of negative harmonic frequencies. Therefore, the C2v conformation with state  is probably a transition structure.
is probably a transition structure.
For Cs conformation with state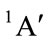 , there are also two of negative harmonic frequencies and it is not possible to be the equilibrium conformation of THF. This would be contrary to Reference [9].
, there are also two of negative harmonic frequencies and it is not possible to be the equilibrium conformation of THF. This would be contrary to Reference [9].
The C1 symmetry with ground state (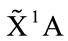 ) is the equilibrium conformation of THF, we believe, for its energy is the lowest one and it is only possible without the negative harmonic frequency. In this conformation the 4 of carbon atoms C and oxygen atom O constitute a curved surface or the helix surface shown in Figure 3(c).
) is the equilibrium conformation of THF, we believe, for its energy is the lowest one and it is only possible without the negative harmonic frequency. In this conformation the 4 of carbon atoms C and oxygen atom O constitute a curved surface or the helix surface shown in Figure 3(c).
In the C2v conformation, 4 of carbon atoms C and oxygen atom O all are on a plane, and for the Cs conformation, 4 of carbon atoms C are on a plane, both of them would be under the much molecular internal stress-strain, because of no sp2 hybridization for each carbon atoms C as described above.

Table 1. The results of theoretical derivation for the possible electronic state of THF.

Table 2. The geometry of C2v and Cs for THF with method (B3P86/6-311G**).
As mentioned before, tetrahydrofuran (THF) molecule (C4H8O) can be as a simple prototype of deoxyribose and has been reviving a quite deal of interest in radiation damage research. It is well known that the sugar ring of deoxyribose is considered as a plane in most of the references such as [21]. If the equilibrium conformation of THF is C1 symmetry with the helix surface, and the sugar ring of deoxyribose would be the helix surface, this
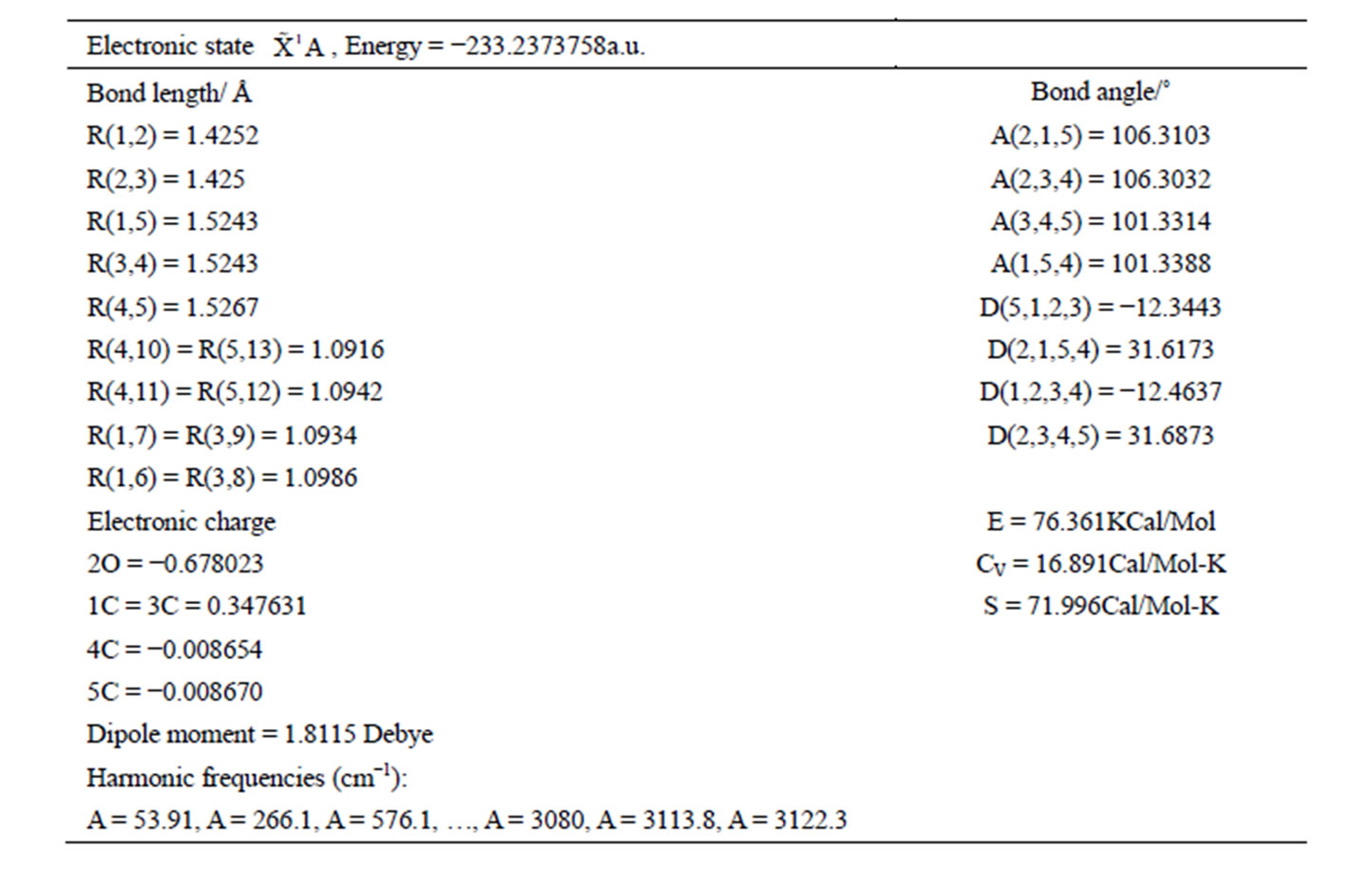
Table 3. The geometry of C1 for THF with method (B3P86/6-311G**).
 (a)
(a) (b)
(b)
Figure 1. The schematic drawing of geometry for THF : (a) Top view; (b) Side view.
: (a) Top view; (b) Side view.
 (a)
(a) (b)
(b)
Figure 2. The schematic drawing of geometry for THF : (a) Top view; (b) Side view.
: (a) Top view; (b) Side view.
probably causes the double helix of DNA. It is hardly believed that the sugar ring is a plane in deoxyribose ring. Many of properties for molecular biology, for example, the sugar moiety itself in DNA being active in the initial molecular processes leading to single strand breaks, have been studied [22], however, there are a few researches on the molecular level.
ACKNOWLEDGEMENTS
This work is supported by the Natural Science Foundation of Hainan (Grant No.110001).
NOTES