Use of Desirability Function Method in Optimization of Regeneration and Callus Induction of Alhagi camelorum ()
1. Introduction
Medicinal plants are of great interest to the researchers in the field of biotechnology as most of the drug industries depend, in part, on plants for the production of pharmaceutical compounds. Among the World’s 25 best selling pharmaceutical medicines, 12 are plant derived [1]. Furthermore, herbs and herbal products are still an important part of the primary health care systems in many parts of the world [2].
Alhagi camelorum (camel thorn) is an invasive, perennial shrub in the Legume family with fairly deep creeping root, 60 to 100 cm long. It is used in Iranian herbal medicine and is known for its antiasthmatic, aphrodiasiac, antipyretic, diuretic, expectorant and laxative effects [3]. Its flowers are used to treat piles, migraine, and warts. Oil from the leaves is used in the treatment of rheumatism [4]. Water extracts of its roots are used to relax the ureter and to remove kidney stones [5]. Chemical investigation of the Alhagi species also revealed the presence of several compounds such as fatty acids and sterols, flavonoids, coumarins, alkaloids and vitamins [6].
Since this plant is drought resistant and contains many beneficial secondary metabolites, the development of an efficient plant regeneration protocol can be a key step for genetic transformation. There is only a few reports about tissue culture of these species. Adventitious bud formation in Alhagi graecorum from callus pathway has reported [7], and Hormonal requirements inducing different regeneration pathways with particular emphasis on somatic embryogenesis in Alhagi graecorum have been studied too [8]. The effects of different concentration of BAP and NAA, LH, GA3 on in vitro propagation and the effects of NAA on seedling rooting had been determined [9]. Hypocotyl tissue culture and plant regeneration of Alhagi were studied [10]. None protocol has yet been standardized for regeneration through callus using a variety of explants in different media. The objective of this research was to evaluate optimum levels of various factors affecting the callus induction, initiation of multiple shoots and rooting of camel thorn. This paper reports the use of desirability function to study the effects of different growth regulators on callus initiation with following shoot development from those calluses on MS and B5 medium supplemented with BAP, NAA and IAA at various concentrations.
2. Materials and Methods
2.1. Preparation of Plant Materials
Mature fruits of Alhagi cameloroum were collected in September from Semnan province, Iran. Then the seeds were removed from fruits with sandpaper. They were brown green and almost enclosed with coat. The seeds were soaked in sulfuric acid 96% for 8 minutes under a laminar flow hood as a dormancy breaking treatment, and then they were surface-sterilized by immersion in 70% (v/v) ethanol for 5 min and rinsed 5 times with sterile water.
2.2. Media and Culture Conditions
Sterilized seeds were cultured on Gamborg medium at 37˚C day/26˚C night, 80% relative humidity, and 16 h 500 mol·m−2·s−1. After 2 weeks seedlings were reached about 15 cm, each with 4 - 5 new nodes (Figure 1). Stems and leaves were then cut into 0.5 cm long segments and 0.5 cm × 0.5 cm pieces; respectively. Approximately 0.5 cm dead tissue at each end of petioles was cut off.
The effects of four factors on callus induction were investigated. These factors were: media (MS, Gamborg), BAP (1,2,3 mg·l−1) and IAA,NAA (2,3,4,5 and 6 mg·l−1), explants (stem and leaf, root) all in 4 treatments . Explants were transferred to Petri dishes containing 20 ml MS or B5 media (Gamborg et al., 1968) with different concentrations of plant growth regulators (BAP, IAA and NAA). The interaction between BAP and both IAA, NAA was also considered. PH was adjusted to 5.8 and the media were solidified with 0.8% agar. The media were autoclaved at 121˚C and 1.06 kg·cm−2 pressure for 15 min. All the cultures were incubated in a growth chamber at temperature of 26˚C ± 1˚C and 16 d/8n photoperiod. Visual observations were made for up to 4 or 5 weeks.
The callus derived from leaves, stems and roots explants were cut into 0.25 cm × 0.25 cm pieces and transferred into the regeneration medium shoot number per explants, shoot length and percentage of regeneration 30 days after culture initiation using 4 replicates for each treatment were analyzed. As elongation treatment of small shoot buds, we used either MS basal medium or
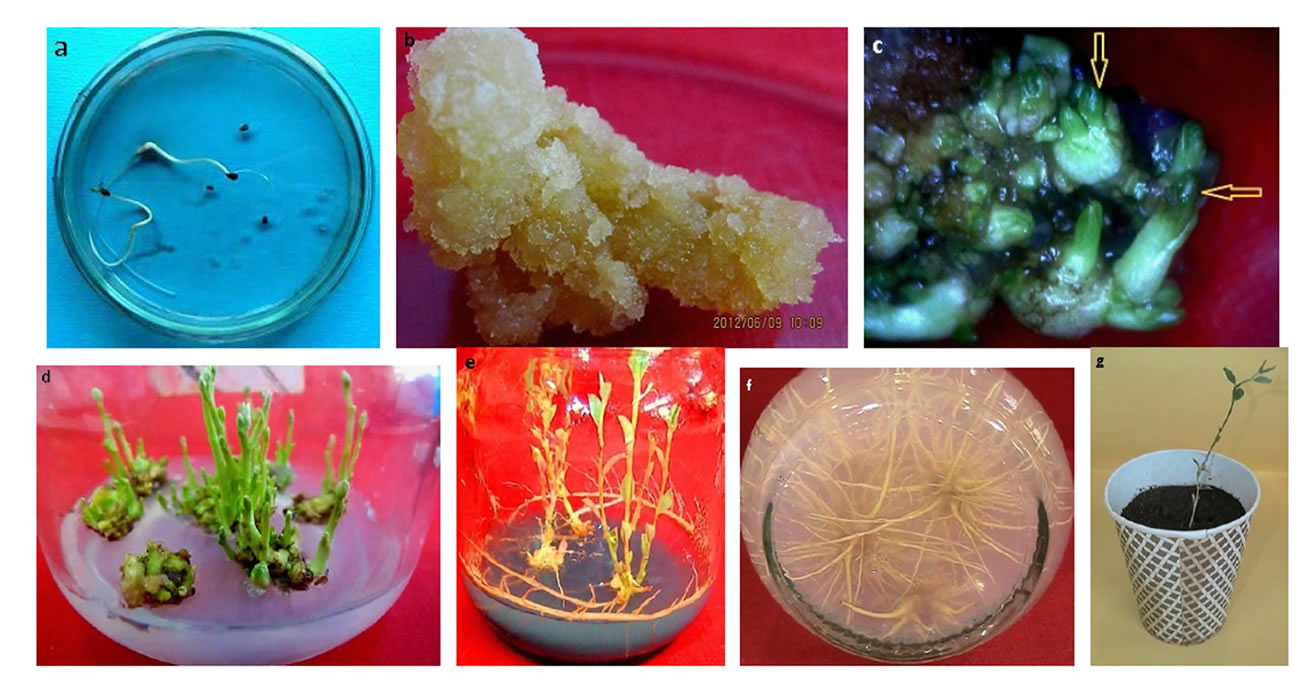
Figure 1. Showing the different steps from callus induction to maturity in Alhagi seed germination in B5 media after 2 weeks. (a) Seed germination; (b) Leaf callus after within 4 weeks of culturing; (c) SEM showing early formation of somatic embryos; (d) Multiple shoots within 4 weeks regenerating callus on shoot generating media after four weeks; (e) rooting of shoot in media supplied with activated charcoal; (f) high number of root in media with lack of AC; (g) Well-rooted Plantlets in the plastic pots for acclimation.
MS medium supplemented with different concentrations of BAP.
Regenerated shoots measuring about 3 cm in length were transferred into the rooting medium. Rooting medium was consisted of three types of medium (MS, half MS and quarter strength MS). As hormonal treatment we used different concentrations of Indole acetic acid IAA (0.25, 0.5, 0.75 and 1 mg·l−1) or A-naphthalene acetic acid NAA (0, 0.5 mg·l−1) alone or in combination with activated charcoal. Percentage of rooting, root number, and root length were recorded after transplanting to the growing mix for 1 month. Plantlets with well-developed roots and shoot systems were transferred into the clay pots containing garden soil and vermiculite in the ratio of 3:1. Acclimatization was not required before transferring the plantlets to the field.
3. Statistical Analysis
In this research we used a full factorial design. Factors affecting regeneration were 2 culture media; MS and B5, 3 explants; leaf, shoot and root and growth regulators. The statistical analysis was performed using one-way ANOVA and goodness of fit was checked too. Each character was analyzed separately and then desirability function was used to determine the optimum conditions for this multivariable system which consequently will allow the optimizing culture media for an adequate experimentation. The programs Design Expert 8 and SPSS 20 were used. The model was designed with the assumption that, initiation of multiple shoots from callus originated from the leaf, shoot and root explants and their subsequent development are function between interacting factors; such as explants, culture media (MS, Gamborg), and growth regulators (BAP, NAA and IAA).
4. Results
The seeds cultured on B5 basal medium, devoid of any plant growth regulator, germinated normally, developed into seedlings with roots and shoots without any callus formation (Figure 1(a)). 100% of callus induction was observed in all explants grown on MS medium supplemented with 2 and 5 mg·l−1 NAA. In the 6 mg·l−1 concentration of NAA, when other factors were varied, maximum fresh weight of callus per culture was 1.7 grams and the maximum dry weight was 0.07 grams.
The color of leaf callus was pinkish white and with compact texture and stem and root callus were yellow cream in color (Figure 1(b)). It was found that the optimum conditions for callus induction included MS medium supplemented with 3 mg·l−1 BAP and 6 mg·l−1 NAA and among three types of explants (leaf, shoot and root), best callus induction was observed in leaf explants (Table 1).
In plantlet development, green spots were observed developing on the embryogenic callus surface after being cultured on regeneration media for 3 - 4 weeks (Figure 1(c)). Subsequently, the green spot developed into green plantlets. Different concentrations of BAP with and without auxins showed various degrees of regeneration. We observed 100% regeneration, maximum of 40 shoot per explant and the maximum shoot length of 7.5 cm (mean) with different concentrations of BAP without auxins. The statistical results showed that all four factors, media, explants, BAP, NAA and IAA significantly (P < 0.05) would influence regeneration percentage (Table 2). As the following table shows, 100% regeneration from root explants were occurred in the medium B5 (BAP of 4 mg·l−1 and NAA 1 mg·l−1), and 100% regeneration from shoot explants happened in the medium B5 contained BAP level 4th and NAA level first (4 mg·l−1 and 0 mg·l−1, respectively), and also in the MS medium with BAP level first and NAA level first (1 mg·l−1 and 0 mg·l−1, respectively). The leaf explants showed 100% regeneration with BAP and NAA in their first levels caused 100%, regardless of the medium used (Table 2). In MS medium, the shoots elongated and thickened more, and became intensively green (Figure 1(d)).
Due to the differential and multiple responses to different studied factors including regeneration percent, shoot length and shoot number per callus we used the desirability optimization methodology (DOM) to transform a multiple response factor into a single factor. Accordingly, the optimum conditions for regeneration were MS medium (A2), BAP 4 mg·l−1 (C4) and the best explants was leaf (B3) (Table 3).
Root induction was achieved using well elongated shoots cultured on 1/4 MS medium supplemented with 0.5 mg·l−1 NAA (Figure 1(e)) and no rooting was observed in full and half strength MS. 100% rooting was observed

Table 1. Desirability function for the optimize condition of callus induction.
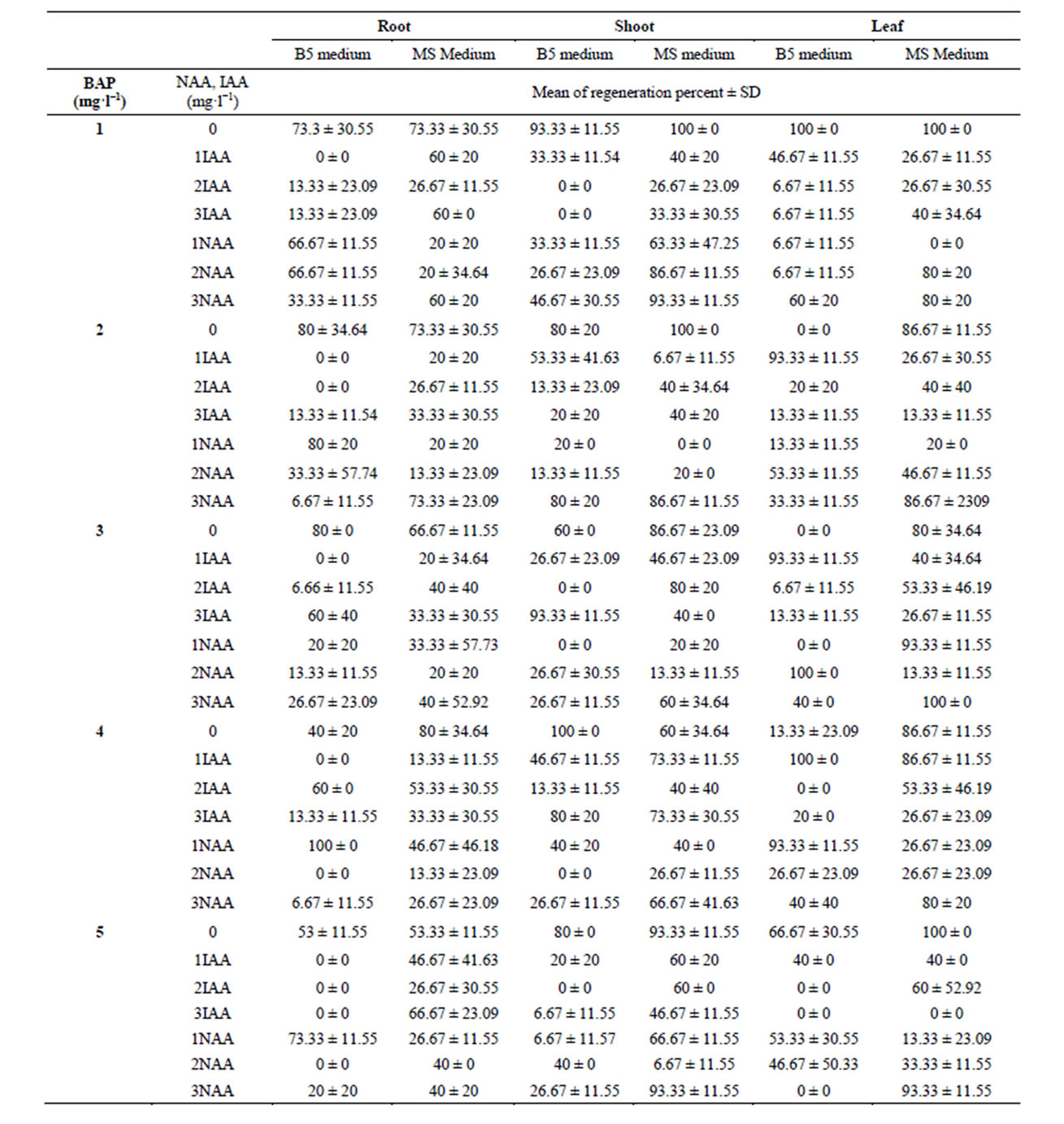
Table 2. Effect of different concentration of BAP, NAA and IAA on regeneration percent in two medium (B5, MS).

Table 3. Desirability function for the optimize condition of regeneration.
on the 1/4 MS medium provided with 0.5 mg·l−1 NAA (Figure 1(f)), the highest number of roots per shoot gained from the medium without activated charcoal (Figure 2(a)) and the longest roots were achieved on the medium containing activated charcoal (Figure 2(b)). The optimum conditions for rooting observed on the medium containing 0.25 mg·l−1 IAA with activated charcoal and no NAA. Regenerated plants were successfully established into complete plants where 70% plantlets were survived (Figure 1(g)).
5. Discussion
5.1. Dormancy Breaking
In order to investigate methods of dormancy breaking in camel thorn seeds, scarification by sandpaper for 2 minutes followed by immersion in 70% (v/v) ethanol for 30 seconds, although there was no infection but germination rate was low. It was found that the most effective treatments for breaking the dormancy in camel thorn was

Figure 2. Effect of activated charcoal and growth regulators on root length. (a) Second treatment (without activated charcoal) results in the highest root number; (b) first treatment (activated charcoal) results in the highest root length.
soaking the seeds in boiling water or scarification by sandpaper, each for 3 minutes under light/dark conditions which resulted in 65% and 55% germination, respectively [11]. This difference can be due to the different conditions in which sandpaper was used. In this study soaking seeds in concentrated sulfuric acid for 8 minutes followed by rinsing with tap water and immersion in 70% ethanol led to best germination rate and least infection rate. Similar results showed soaking A. sparsifolia seeds in concentrated sulfuric acid shortens seed germination from 1 to 4 days [12]. Sulfuric acid also could break dormancy of Alhagi sp. [13], so it is possible that Sulfuric acid removes seedling emergence inhibitors from the seed coat of A. sparsifolia, and then breaking of dormancy occurs.
5.2. Callus Induction
A stimulation of callus growth was observed on hypocotyls segments cultivated on the MS medium contained both BAP (3 mg·l−1) and NAA (6 mg·l−1), as compared to the medium supplemented with IAA. A strong callus induction of the Alhagi was observed on the MS medium. Callus segments also were obtained on MS medium supplemented with 0.56 mg·l−1 BAP, 0.93 mg·l−1 1-naphthaleneacetic acid (NAA) and 0.11 mg·l−1 2,4-dichlorophenoxyacetic acid (2,4-D) [14]. In addition, small callus size has been reported to be an important factor in absorption of nutrients and proliferation of callus in wheat [15]. So to improve callus growth and embryogenic callus formation, we divided the callus into small pieces (50 mg) for each subculture.
5.3. Organogenesis
MS medium supplemented with 4 mg·l−1 of BAP enhanced the shoot regeneration rate but further increase in BAP (5 mg·l−1) concentration did not provide any advantage. This shows the positive effect of BAP on shoot growth and shoot multiplication. As in earlier studies, BAP was proved to be the most effective cytokine for shoot multiplication in many other plants like Hymenocallis littoralis [16], Ficus anastasia [17], and sugerbeet [18]. In general, the best response was observed with MS medium for shoot proliferation rate, which might be due to its higher nitrogen content. It is clear that there is strong interaction between macro elements and BAP present in the culture media in Alhagi. It was reported that the effect of growth regulators can be strongly modified by the medium on which the culture is grown [19]. The interaction indicated that the most appropriate mineral salt medium was MS (high in nitrogen and potassium). It was reported that nitrogen can function as a signal molecule for plant growth via increasing gene expression of the enzyme responsible for uptake and utilization of nitrate [20]. Our results are also in accordance with the study that showed shoot formation from callus in Alhagi graecorum happened when it was transferred to MS medium supplemented with 0.56 mg·l−1 BAP [7].
Based on this experiment, the presence of activated charcoal (AC) in the rooting medium increased the root length (up to 5.50 cm) but reduced the root numbers (20% - 25%). This is in agreement with an earlier report that showed activated charcoal could suppress callus formation [21]. Activated charcoal absorbs inhibitory substances accumulated in the culture medium and thus is often used to reduce the oxidation of phenolic compounds in tissue culture in order to increase cell growth and development [22]. Moreover, no rooting was observed in full and half strength MS; but 1/4 MS salt yielded a suitable result. So rooting in Alhagi is positively influenced by low concentrations of minerals and appropriate culture media for initiation and elongation of the roots.
In conclusion, we believe, in this study for the first time, we established an efficient plant regeneration system for A. camelorum, which can offer an effective approach to achieve a rapid propagation, and to provide a foundation for its genetic transformation attempts in the future.
Abbreviations
MS: Murashige and Skoog (1962) medium BAP: 6-Benzyladenine IAA: Indole-3-acetic acid NAA: Naphthaleneacetic acid
NOTES