Immune Control of Equine Infectious Anemia Virus Infection by Cell-Mediated and Humoral Responses ()
1. Introduction
One of the most significant gaps in our knowledge of virus-host dynamics involves how different immune responses work together to counteract the pathogen. Understanding viral dynamics in the context of immune responses is essential for advancing our knowledge of host-pathogen interactions as well as for developing control strategies. Mathematical modeling has been instrumental in the study of viral dynamics and has increased our understanding of basic pathogenic interactions for infections including human immunodeficiency virus, hepatitis B virus, hepatitis C virus, and influenza virus [1-6]. In this paper, we study Equine Infectious Anemia Virus (EIAV).
EIAV is a retrovirus of the genus lentivirus that infects equids such as horses and ponies. EIAV is spread between horses through biting flies [7]. Horse flies, primarily of the family Tabanidae [8], feed on acutely infected horses and spread the virus through a subsequent blood meal on an uninfected equid. Due to this insect vector, EIAV tends to be concentrated in warmer climates, but is considered a worldwide infection [7,9]. To control the spread of infection, horses are routinely tested at racetracks, shows, and rodeos, before breeding, and crossing borders. This effort has proven largely successful, bringing the prevalence of EIAV-positive animals down to 0.38% by 1988 [7,10].
EIAV targets monocyte-derived macrophages in several tissues of infected equids, including spleen, liver, lungs, and bone marrow [7,11]. These tissues serve as reservoirs for infection for the remainder of the animal’s life. Immune control of EIAV is attributed to both cytotoxic T lymphocyte (CTL) [12,13] and broadly neutralizing antibody (bnAb) [14] activity. Due to the changing dynamics of both the CTL and bnAb responses in both specificity and timing, it is believed that both responses are essential for long-term control of the infection.
Infection with EIAV typically follows three stages: acute, chronic, and asymptomatic [7]. The acute stage is an initial febrile episode associated with the onset of infection, high viral titer, and an adaptive immune response. Once the initial infection is brought under control, antigenic variants escape the immunological control and cause the increases in viral load and fevers associated with the chronic stage. After six to twelve months, the recurrent fevers cease and the animal enters the asymptomatic stage, which is associated with very low viral load and the absence of clinical symptoms. In some cases, a fourth recrudescent stage is seen, where the infected animal experiences recurring fevers for the remainder of its life. This stage is often associated with immunosuppressed or otherwise immunocompromised animals [15].
EIAV shows many life history traits similar to other lentiviruses, including a very rapid replication rate and high levels of antigenic variation. However, EIAV is atypical among lentiviruses in that most infected animals experience a few episodes of fever and high viral titer and then progress to the asymptomatic stage characterized by low viral titer and an absence of clinical disease manifestations. This is in direct contrast to lentiviruses human immunodeficiency virus (HIV) and simian immunodeficiency virus (SIV), in which infected individuals and animals develop immunodeficiency and disease, and makes EIAV an especially interesting comparison species for both clinical research and mathematical models [16].
There is a great body of previous work modeling the dynamics of viral infection. The standard three equation model of viral infection considers the uninfected cell, infected cell, and virus populations but does not include the dynamics of immune compartments [16-18]. Subsequent models augmented the standard model to four or five equations, examining the dynamics of the cellular (CTL) and humoral (antibody) immune responses individually [17,19] and in concert [20]. Two studies explicitly modeled viral dynamics with the dynamics of CTLs and antibodies to study Hepatitis C Virus (HCV) [21,22]. Wodarz investigated the dynamics and pathology of HCV, and Yousfi et al. extended the theoretical results by Wodarz with a global stability analysis. However, these studies used a model with a depiction of antibody production in which antibodies proliferate according to mass action between antibodies and virus; this depiction is imprecise and a more accurate representation is needed.
The goal of this study is to create a mathematical model of EIAV and immune system dynamics in order to predict conditions that correlate with viral control. We use a five-equation model, explicitly containing the dynamics of CTLs and of antibodies, which builds upon previous work. We include an alternate equation for antibody dynamics that models antibody production in direct proportion to virus, consistent with known immunology [23]. We use our model to gain insight into EIAV infection, in which both CTL and antibody responses are known to be important for control. Specifically, our analysis gives the characteristics of 3 scenarios: no infection, viral persistence without CTLs, and viral persistence with both CTL and antibody responses. We simulate the longterm dynamics of viral infection, showing viral persistence in the context of antibodies alone or of both antibodies and CTLs.
This paper is organized as follows: Section 2 contains a description of the deterministic model along with its equilibrium points. In Section 3 we discuss the linear stability analysis of the biologically relevant equilibria and the conditions for their stability. In addition we discuss the basic reproduction number, 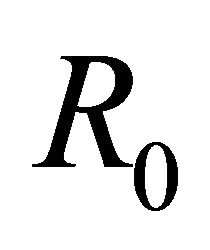 , of the model. We also provide numerical simulations that illustrate the behavior of solutions for representative parameter sets. In Section 4 we conclude the paper with a discussion of the implications of these results for understanding control of EIAV infection by the cell-mediated and humoral arms of the immune response.
, of the model. We also provide numerical simulations that illustrate the behavior of solutions for representative parameter sets. In Section 4 we conclude the paper with a discussion of the implications of these results for understanding control of EIAV infection by the cell-mediated and humoral arms of the immune response.
2. Mathematical Model
In this section we present a five-equation model that builds upon earlier studies [16,17,21,22]. Our model explicitly contains the dynamics of CTLs and antibodies, including an equation for antibody dynamics that models antibody production in direct proportion to virus [23]. We then find the steady state solutions.
2.1. Model Formulation
Our deterministic model representing the five interacting populations is shown by the following system of ordinary differential equations:

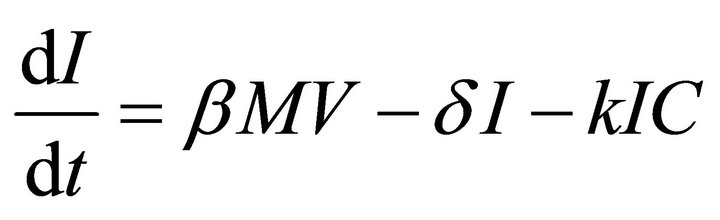
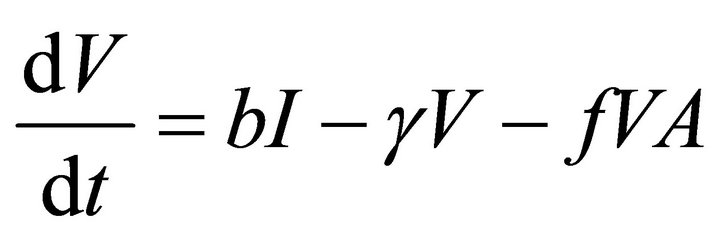
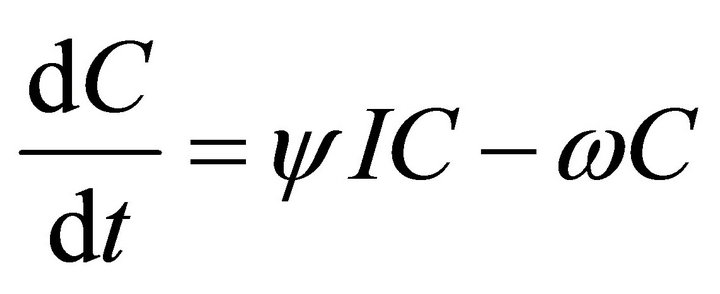

In this model, the target cells of EIAV infection are monocyte-derived tissue macrophages [7,11,24,25]. The number of uninfected target cells is represented by M. These cells become infected cells (I) following contact with virus (V) at rate . Uninfected target cells are generated at rate
. Uninfected target cells are generated at rate  and die at rate
and die at rate . The infected cell death rate is
. The infected cell death rate is . Infected cells are killed by cytotoxic T lymphocytes, or CTLs (C), at rate
. Infected cells are killed by cytotoxic T lymphocytes, or CTLs (C), at rate . Virus is produced by infected cells at rate b and cleared at rate γ. The virus is neutralized by antibodies (A) at rate
. Virus is produced by infected cells at rate b and cleared at rate γ. The virus is neutralized by antibodies (A) at rate . CTLs proliferate in response to contact with infected cells at rate
. CTLs proliferate in response to contact with infected cells at rate  and die at rate
and die at rate . The antibody population grows in proportion to virus at rate α [23] and is cleared at rate
. The antibody population grows in proportion to virus at rate α [23] and is cleared at rate . A schematic diagram of the model dynamics, indicating the flow in and out of each compartment, is shown in Figure 1. Model variables and parameters are listed in Table 1. The initial conditions for the model are
. A schematic diagram of the model dynamics, indicating the flow in and out of each compartment, is shown in Figure 1. Model variables and parameters are listed in Table 1. The initial conditions for the model are  and
and 
 . We assume all parameters are nonnegative.
. We assume all parameters are nonnegative.
2.2. Equilibrium Points
The equilibria (or steady states) of the model are found by setting the equations of the model to zero. The infection-free steady state (also called the infection-free equilibrium, or IFE) is given by

We obtain four other solutions: and
and , where
, where


Figure 1. Schematic diagram of mathematical model of EIAV infection with cellular and humoral immune responses. Populations modeled include the target cells (macrophages, M), infected cells (I), virus (V), cytotoxic T lymphocytes (C) and antibodies (A).
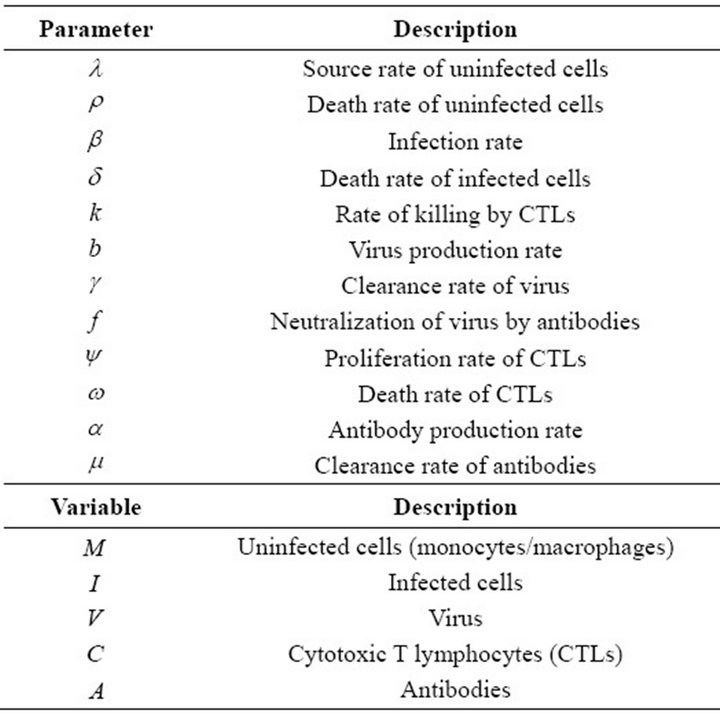
Table 1. Parameters and variables of model of EIAV infection.
Since  and
and  are less than zero, the steady states
are less than zero, the steady states  and
and  are not biologically meaningful and therefore will not be discussed further.
are not biologically meaningful and therefore will not be discussed further.
3. Linear Stability Analysis of Model Equilibria
In this section we discuss the basic reproductive number,  , which arises from linear analysis around the infection-free equilibrium point,
, which arises from linear analysis around the infection-free equilibrium point, . We provide stability conditions for
. We provide stability conditions for 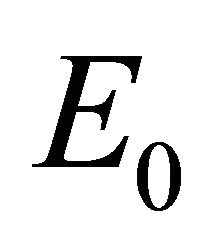 in Theorem 2, and we provide existence criteria for
in Theorem 2, and we provide existence criteria for  and
and  in Theorem 3. In addition we provide a numerical stability analysis as well as numerical simulations that illustrate the stability of these equilibrium points.
in Theorem 3. In addition we provide a numerical stability analysis as well as numerical simulations that illustrate the stability of these equilibrium points.
3.1. Analytical Results
The basic reproductive number,  , is a threshold that delineates whether an infection spreads or dies out when a single infected cell encounters a population of uninfected target cells [26-30]. If
, is a threshold that delineates whether an infection spreads or dies out when a single infected cell encounters a population of uninfected target cells [26-30]. If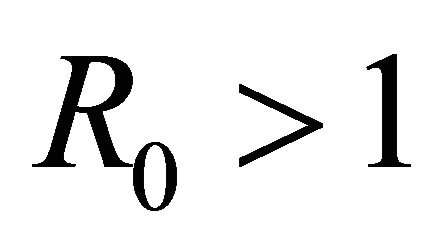 , then more than one cell (on average) becomes infected and the infection will spread; if
, then more than one cell (on average) becomes infected and the infection will spread; if , then less than one cell (on average) becomes infected and infection will not take hold [31].
, then less than one cell (on average) becomes infected and infection will not take hold [31].
Theorem 1. The basic reproductive number of the model is .
.
Proof. We determine  using the next-generation method [32]. Consider the matrix of new infections,
using the next-generation method [32]. Consider the matrix of new infections, 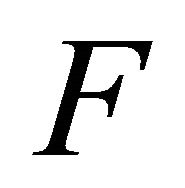 , and the matrix of transfers,
, and the matrix of transfers,  , both evaluated at the infection-free equilibrium:
, both evaluated at the infection-free equilibrium:
 and
and . Hence,
. Hence, .
.
The basic reproductive number is given by the spectral radius (i.e., the eigenvalue with the largest modulus) of
 . Hence,
. Hence, .
.
Theorem 2. The IFE,  , of the model is linearly asymptotically stable and attracting for
, of the model is linearly asymptotically stable and attracting for  and unstable for
and unstable for .
.
Proof. That the IFE is linearly asymptotically stable and attracting for  but unstable for
but unstable for 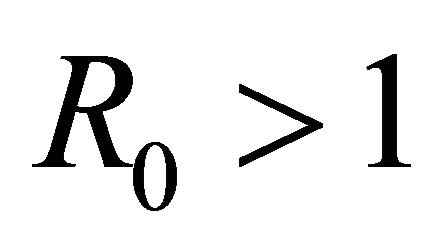 is an immediate consequence of the computations provided in Theorem 1 and the results of van den Driessche and Watmough [32].
is an immediate consequence of the computations provided in Theorem 1 and the results of van den Driessche and Watmough [32].
The equilibrium  exists if
exists if  is biologically relevant, i.e.,
is biologically relevant, i.e.,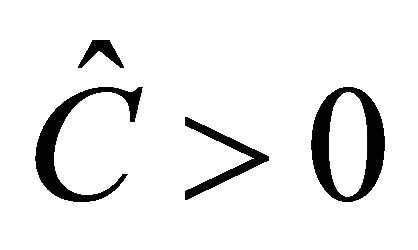 .
.  can be rewritten as
can be rewritten as , where
, where . Hence,
. Hence,  exists if
exists if .
.
We will refer to 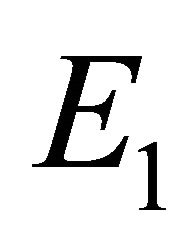 as the boundary equilibrium, which represents the case with the presence of antibodies and without CTLs. We will refer to
as the boundary equilibrium, which represents the case with the presence of antibodies and without CTLs. We will refer to  as the endemic equilibrium, which represents the case with the presence of both antibodies and CTLs.
as the endemic equilibrium, which represents the case with the presence of both antibodies and CTLs.
Theorem 3. If , then
, then 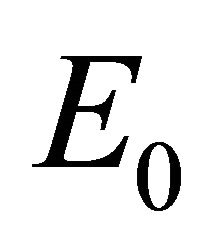 is the only equilibrium point and is linearly stable. If
is the only equilibrium point and is linearly stable. If , then
, then  is unstable, and boundary equilibrium
is unstable, and boundary equilibrium  exists. If
exists. If , then
, then  is unstable, boundary equilibrium
is unstable, boundary equilibrium  exists, and endemic equilibrium
exists, and endemic equilibrium 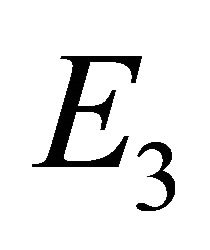 exists.
exists.
Proof. We obtain that  from the inequalities
from the inequalities
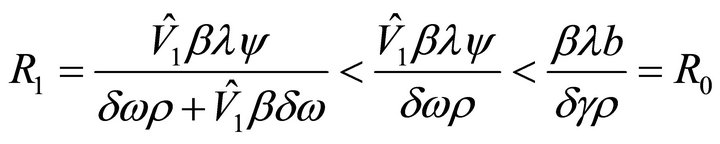
since . The latter inequality is shown as follows.
. The latter inequality is shown as follows.
In determining the equilibrium points  and
and , the values
, the values  and
and 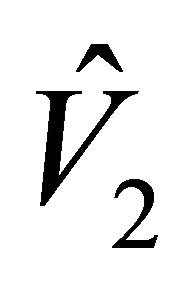 were found as the roots of the quadratic function
were found as the roots of the quadratic function
 .
.
Since the leading coefficient of  is positiveand
is positiveand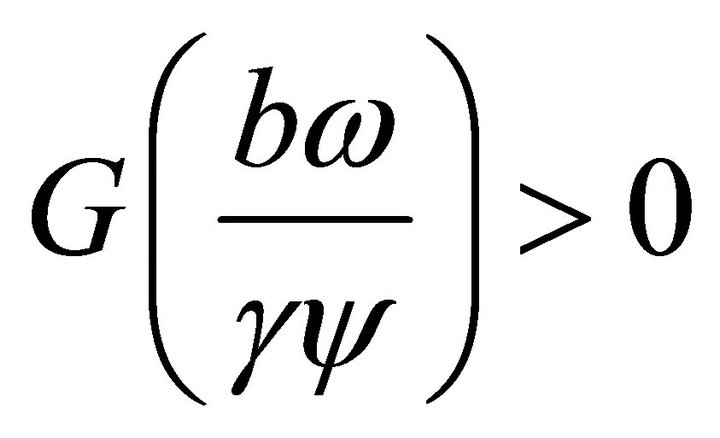 , then the positive root
, then the positive root  lies to the left of the positive value
lies to the left of the positive value . That is,
. That is,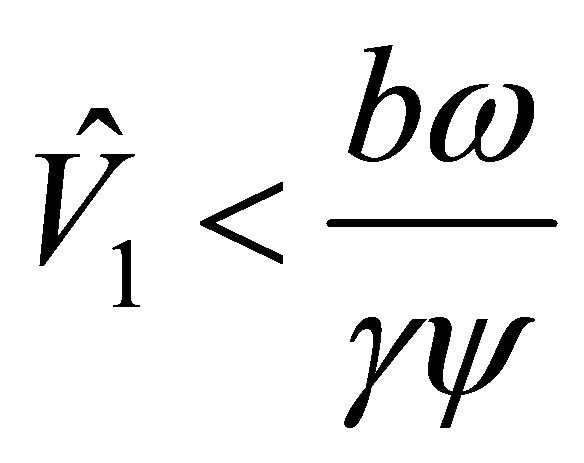 .
.
We now consider the existence of the equilibrium points  and
and . This is determined by the sign of the roots
. This is determined by the sign of the roots  and
and 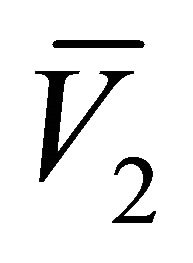 of the quadratic function
of the quadratic function
 .
.
Since , then if
, then if , we obtain
, we obtain

which implies that  and
and . Hence, the equilibrium points
. Hence, the equilibrium points 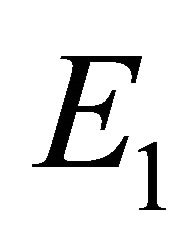 and
and  are not biologically possible. If
are not biologically possible. If , we obtain
, we obtain

which implies that  and
and . This indicates that
. This indicates that  is an existing equilibrium.
is an existing equilibrium.
The endemic equilibrium  exists only if
exists only if .
.
Since ,
,  is biologically possible only if
is biologically possible only if . Theorem 3 follows from the arguments above.
. Theorem 3 follows from the arguments above.
We note that  is a threshold that delineates the boundary equilibrium
is a threshold that delineates the boundary equilibrium 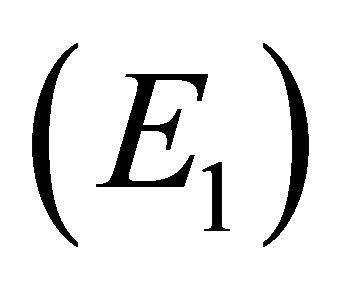 from the endemic equilibrium
from the endemic equilibrium .
.
3.2. Numerical Stability Analysis
In the previous sections we found the steady states of the model and determined thresholds for their linear stability. The biologically plausible steady states ,
,  , and
, and  represent the scenarios of the infection-free equilibrium (IFE), the boundary equilibrium with no CTLs, and the endemic equilibrium with both CTL and antibody function (Ab), respectively.
represent the scenarios of the infection-free equilibrium (IFE), the boundary equilibrium with no CTLs, and the endemic equilibrium with both CTL and antibody function (Ab), respectively.
We next performed a linear stability analysis of the equilibria of the model by using numerical values for the parameters (i.e., a unique parameter set representing each of the three equilibria) and determining the eigenvalues of the Jacobian matrices evaluated at each steady state. Negative real parts of all the eigenvalues indicated stability of the steady state. We present the results using three representative parameter sets. Our results are summarized in Table 2. Parameter sets 1, 2, and 3 were selected because they predict equilibria ,
,  , and
, and , respectively. These parameter sets differ in the value for λ; equivalent results were found by altering the other parameters that affect
, respectively. These parameter sets differ in the value for λ; equivalent results were found by altering the other parameters that affect  (data not shown).
(data not shown).
3.3. Numerical Simulations
We now illustrate the results on linear stability with numerical simulations using each of the three parameter sets.
Infection-Free Equilibrium, E0. In the IFE, the infection dies out. All populations approach zero except the number of uninfected cells, which approaches its uninfected steady state level. The time course of infection showing each of the populations over 150 days is shown in Figure 2.
Boundary Equilibrium, E1. The boundary equilibrium  represents the case with antibodies but no CTLs. Here
represents the case with antibodies but no CTLs. Here  and
and . The long-term dynamics show that all populations reach a positive steady state except CTLs, which decay to zero. The time course of infection showing each of the populations over 150 days is shown in Figure 3.
. The long-term dynamics show that all populations reach a positive steady state except CTLs, which decay to zero. The time course of infection showing each of the populations over 150 days is shown in Figure 3.
Endemic Equilibrium, E3. The endemic equilibrium  shows the case with both antibodies and CTLs. We observe nonnegative steady state values for all populations. In this case,
shows the case with both antibodies and CTLs. We observe nonnegative steady state values for all populations. In this case,  and
and . The time course of infection, showing each of the populations over 150 days, is shown in Figure 4. This is the case that correlates with clinical infection of horses with EIAV.
. The time course of infection, showing each of the populations over 150 days, is shown in Figure 4. This is the case that correlates with clinical infection of horses with EIAV.
Long-term dynamics over 3000 days. Equilibrium values are seen clearly in simulations run over 3000 days. The boundary equilibrium over 3000 days is shown in Figure 5. The endemic equilibrium over 3000 days is shown in Figure 6. We observe that final values for ,
,  ,
,  and
and  are greater in
are greater in  than in
than in , but this is likely due to the larger value for
, but this is likely due to the larger value for 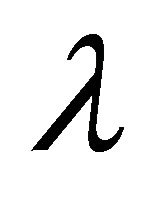 in this parameter set. For parameter set 1 representing the IFE, all populations except
in this parameter set. For parameter set 1 representing the IFE, all populations except 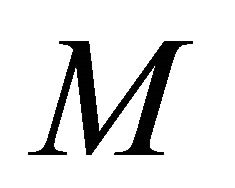 reach zero over 3000 days (data not shown).
reach zero over 3000 days (data not shown).
4. Discussion
In summary, we constructed a mathematical model of EIAV infection that takes into account the dynamics of cell-mediated and humoral immune responses. Both of these immune components have been found to be necessary for immune control of this infection. We performed linear stability analysis and simulation of the model to predict long-term behavior in healthy and infected states. We presented equations for the basic reproductive number  as well as a second threshold,
as well as a second threshold,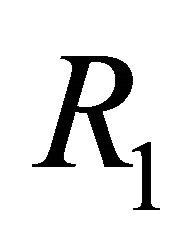 . We showed that
. We showed that  distinguishes the IFE
distinguishes the IFE  from the boundary equilibrium
from the boundary equilibrium  and the endemic equilibrium
and the endemic equilibrium , and that
, and that  distinguishes
distinguishes  from
from . Finally, we presented parameter sets that correlate with the results of the linear stability analysis.
. Finally, we presented parameter sets that correlate with the results of the linear stability analysis.
The steady states each describe a scenario with a different virological and immunological profile: viral clearance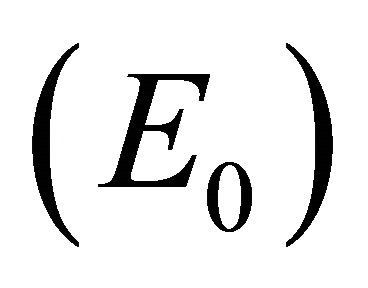 , control of infection with antibodies and no CTLs
, control of infection with antibodies and no CTLs , and control of infection with both antibodies and CTLs
, and control of infection with both antibodies and CTLs . Biologically, two of these scenarios are seen in horses: viral clearance (i.e., no infection)
. Biologically, two of these scenarios are seen in horses: viral clearance (i.e., no infection) 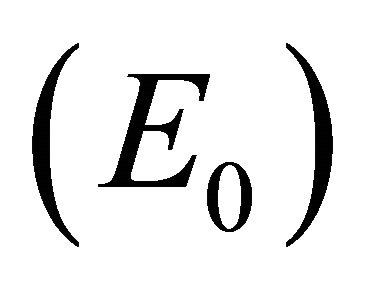 and control of infection with both antibodies and CTLs (i.e., coexistence of all five populations)
and control of infection with both antibodies and CTLs (i.e., coexistence of all five populations) . Since coexistence is what is observed in clinical EIAV infection, knowledge of the characteristics of this steady state may be useful both for understanding fundamental mechanisms of immune control as well as for developing therapeutic strategies to bring about the control of viral
. Since coexistence is what is observed in clinical EIAV infection, knowledge of the characteristics of this steady state may be useful both for understanding fundamental mechanisms of immune control as well as for developing therapeutic strategies to bring about the control of viral

Table 2. Numerical results of linear stability analysis.

Figure 2. Long-term dynamics of M, I, V, C, and A populations over 150 days for a parameter set representing the infection-free equilibrium (IFE). Parameters are as follows: λ = 0.05, ρ = 0.01, β = 0.0001, δ = 0.5, k = 0.01, b = 10000, γ = 20, ψ = 0.75, ω = 5, f = 3, α = 150, μ = 20 (parameter set 1). Here, R0 = 0.5 and R1 = 0.

Figure 3. Long-term dynamics of M, I, V, C, and A populations over 150 days for a parameter set representing the E1 boundary equilibrium. Parameters are as in Figure 2 except λ = 1 (parameter set 2). Here, R0 = 10 and R1 = 0.02.

Figure 4. Long-term dynamics of M, I, V, C, and A populations over 150 days for a parameter set representing the E3 endemic equilibrium. Parameters are as in Figure 2 except λ = 50 (parameter set 3). Here, R0 = 500 and R1 = 5.26.

Figure 5. Long-term dynamics of M, I, V, C, and A populations over 3000 days for parameter set 2 representing the E1 boundary equilibrium. Parameters are as in Figure 3.

Figure 6. Long-term dynamics of M, I, V, C, and A populations over 3000 days for parameter set 3 representing the E3 endemic equilibrium. Parameters are as in Figure 4.
infection without disease. Future work in this area may be applicable for understanding other lentiviral infections that cause disease, such as HIV.
5. Acknowledgements
The authors would like to thank Pauline van den Driessche for insightful comments on the manuscript.
NOTES