1. Introduction
With expansion of the agriculture areas with high biodiversity are increasingly being devastated, causing severe assaults on ecosystems. A consequence of this process is the reduction of the population of pollinating insects, which are of utmost importance for the maintenance of the environment. Therefore, the agricultural pollination should be treated as an important factor of production and its approach should not be limited to general concepts. There is a need to study aspects of pollination of each crop [1].
Data on the viability pollen grains and of growth of pollen germinability are key to studies about reproductive biology and breeding, as they allow greater safety to processes conducted in order to generate new hybrids and/or increase the viability and fruit production [2].
According to Viana et al. [3], with studies on the pollination it is possible to obtain an increase in the levels of fruit production, as regards the quality and size. This enables greater economic feedback to producer. So that studies of germination and pollen viability are tools to fruit production, because of rate of germinated pollen grains is directly related to the amount of fruits and seeds. Gaps in that subject are a great is a problem for agriculture.
The species Malpighia emarginata DC., West Indian cherry (Malpighiaceae family), focused here, is important because of its role in economy, mainly in familiar economy. It generates income for many families in urban areas where it is cultivated in because of its juicy fruit, rich in vitamin C and antioxidants components. This is an exotic plant to Brazilian flora, but it is present in many home backyards and small farms because of its juicy red fruits.
The fruits of M. emarginata have high nutritional value, which is due to its high content of vitamin C-1000 - 4676 mg per 100 g of pulp, 30 - 50 times higher than that found in orange [4]. Ascorbic acid (vitamin C) is essential for maintaining the immune system as it fights infections, and assists in restoring tissue and still be a potent antioxidant [5].
During the last years, pollen grains have been used as biological model for physiological studies, due to their simple structure, and their highly sensitiveness to environmental factors, particularly temperature and humidity [6].
Among Angiosperms, the initial stage of pollen grain germination is its hydration and activation of its metabolism, which provide a rapid growth of the pollen tube through stigma and style toward the ovary, where fertilization occurs [7]. During the first minutes of contact with the surface of the stigma, or any other medium the pollen grain releases peptides and proteins diffused from the cell wall and from pollenkitt (a lipophilic layer covering the pollen wall) [8]. In the course of metabolic activation, pollen grain begins to excrete compounds produced by protoplast. Among the proteins released by the pollen grains are enzymes, such as the β-expansins and also proteins and peptides that control the recognition of the stigma, hydration and pollen tube growth [7,9].
According to Rudiger and Gabius [10], it is unknown the presence of lectins in the wall of the pollen grain. Lectins are proteins that specifically interact with carbohydrates, but not modify them and are molecules that have one or several binding sites with their oligosaccharides. Lectins produce three-dimensional networks of crosslinked glycoconjugates, which allow their detection, due to the cellular precipitation or clumping [4]. According Salles et al. [2], in a study with in vitro pollen germination of Citrus, pollen tube growth is stopped before it gets the size usually reached in the stigma, because of the medium composition and pH-factors that affect the formation of the pollen tube.
This study consists of the search of enlightenment involving the theme of germination of pollen grains, specifically with regard to the action of lectins on the germination of pollen grains of Malpighia emarginata (Malpighiaceae) in relation to the formation of the pollen tube. The literature provides a diversity of biological functions of lectins in plants but few considering pollen germinability [11-13]. We used assessments of pollen germinability with CPL lectin. It is a lectin isolated from the seeds of Crotalaria pallida L. (Leguminosae) [14], following statements of the study of Southworth [11], who pioneered the use of exogenous lectins in the formation of the pollen tube.
2. Material and Methods
2.1. Flowers and Pollen Grains Collect
Flowers were collected from trees in the experimental orchard West Indian cherry (Malpighia emarginata, Malpighiaceae) Centro Territorial de Educação Profissional do Portal do Sertão (CETEP, Feira de Santana, Bahia, Brazil). Pollen was collected from pre-anthesis flowers. The flowers were collected and disposed to dry in a box with filter paper and silica gel, at room temperature (25˚C - 27˚C) for 72 hours. Samples were collected in three batches (called here as samples) for tests of pollen viability and germinability.
2.2. Assessment of Pollen Viability
Pollen viability was assessed using aniline blue in lactophenol staining, according with method stated by Kears & Inouye [15]. Pollen grains of Malpighia emarginata were scattered in a drop of stain mixture. Viable pollen grains turned blue, while non-viable pollen remained uncolored. In order to statistics analysis, 1.000 pollen grains were counted by each sample.
The Sudan IV stain was used in order to verify the chemical nature of material on exine (wall) of the pollen grains of M. emarginata [16].
2.3. Purification of CPL Lectin
In order to have CPL lectin to use in treatments, mature seeds of Crotalaria pallida (50 g) were ground, and the proteins were extracted in saline solution and acetone (80%, v/v) fractionation. The lectin was purified by gel filtration on Sephadex G-75 (0.05 M Tris-HCl buffer, pH 8.0, containing 0.15 M NaCl), and HPL Canion exchange on DEAE-5PW column (0.05 M Tris-HCl buffer, pH 8.0 with a linear NaCl gradient of 0 - 5 mM). After purification, the lectin was dialyzed extensively against distilled water and lyophilized [14].
2.4. Assessment of in Vitro Pollen Germinability
For in vitro germinability test, it was used hanging-drop method, with a solution of 1% agar plus sucrose 10%, boric acid 0.01% and calcium nitrate 0.03%. Pollen grains were placed to grow pollen tube in this medium.
The concentration of sucrose was determined in advance by a previous study in which a liquid medium was tested with different concentrations of the sugar (5%, 10%, 20%, 30% and 40%). This medium also contained boric acid and calcium nitrate in concentrations commonly used in the specific literature [17-22]. This prior test indicated that sucrose concentrations of 10% and 20% are the best to deal with germinability test, because they presented best result regarding amount of germinated pollen grains. Both sucrose concentrations presented results statistically equivalent, so the one of 10% was adopted as a matter of parsimony in the use of carbohydrate.
Pollen germination test was performed on slides in a wet chamber maintained in Petri dishes, which were left at room temperature (25˚C - 27˚C) for 12 h and up to 24 h. Pollen grains were placed to germinate in treatments tested (see below), and after respective incubation times were counted pollen grains which have germinated on a randomly sample of 500 grains in three slides by each treatment. For the purpose of counting, germinated pollen was considered when the pollen tube length was equal to or more than twice the diameter of the each pollen grain.
In order to analysis of action of CPL lectin [14], three treatments were made in which the pollen grains were placed to germinate (in vitro):
T0—control test, no addition of lectin, it was used the pure medium;
T1—to the germination medium was added 1.0 µg/ml of CPL lectin;
T2—to the germination medium was added 3.0 µg/ml of CPL lectin.
The experimental design was completely randomized. Pollen grains data were analyzed by One-Way ANOVA (Analysis of variance with one factor), with 95% reliability and comparison of means by Turkey test at 5% probability.
3. Results
Pollen viability assessed by staining method by aniline blue in lactophenol revealed high values in the three samples of pollen grains of Malpighia emarginata (Table 1), with a figure around 68%. Non viable pollen grains are usually smaller than viable ones (Figure 1(A)).
During microscope pollen observation, the presence of fatty material on pollen wall was noticed (Figure 1(B)), its chemical nature (lipid) was detected using Sudan IV, a stain specific to oil and fat.
Despite the high rate of viability, pollen grains showed a germinability (formation of the pollen tube) that did not exceed 24% (Table 2). Pollen germinability was more expressive among pollen grains that were subjected to long time germination and also to a higher concentration of CPL lectin. Thus, the higher germinability were reached by the pollen grains growth for 24 h treatment T2- concentration of 3.0 µg/ml CPL lectin (22.267%) (Table 2). The lowest germinability rates were observed in pollen germinated for 12 hours without added lectin CPL (T0).
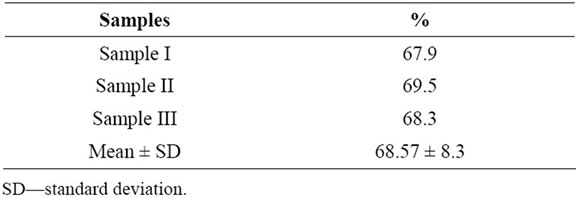
Table 1. Pollen viability of Malpighia emarginata assessed with aniline blue in lactophenol staining.

Figure 1. Germination of pollen grains of Malpighia emarginata (Malpighiaceae). A) Viability test, viable grains are dark and non viable ones are clear; B) pollen gains with oilpollenkitt (arrows); C) and D) germinated pollen grains in medium with addition of 3.0 µg/ml of CPL lectin, notice long pollen tube (D) (Scale bar = 10 µm).
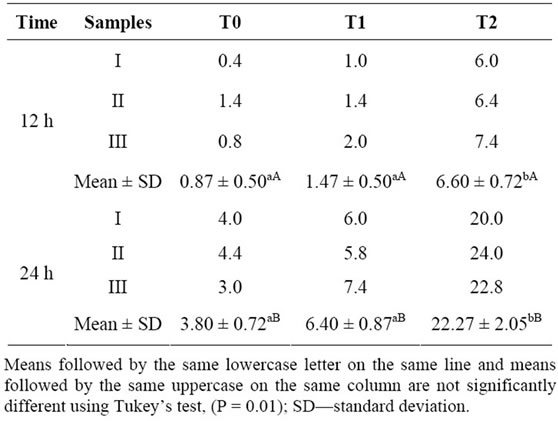
Table 2. Germinability of pollen grains of Malpighia emarginata assessed with different concentrations of CPL lectin added to the growing medium: T0—control, without lectin; T1—with 1.0 µg/ml; T2—with 3.0 µg/ml.
Pollen tube were observed in all treatment, in T0 and T1 rare pollen grains presented developed pollen tube (at least as long as twice pollen diameter). On the other hand, pollen grains in T3 presented long pollen tubes (Figures 1(C) and (D)).
Statistical analysis (ANOVA) showed that the germinability obtained with the medium without lectin (T0) and with addition of 1.0 µg/ml (T1) did not have significant differences, although they exhibit different values, however these are statistically lower than the obtained in the treatment T2 (Table 2). These results indicated that there is a minimum concentration necessary of CPL lectin to increase the process of pollen tube growth in Malpighia emarginata. Analyzes also indicated that there was an influence of time on germinability, the longer the time is, the higher is the germinability of pollen grains.
4. Discussion
Pollen grains from flowers of Malpighia emarginata have a wide range of viability of 10% to 90% [23]. The decrease in pollen viability rate, which is often observed, may be related to the loss of water permeability by intine during pollen storage pollen, with subsequent reduction of hydration [24]. Thus, considering that here we used pollen grains from flowers freshly collected, the protocols used for the viability and germinability should be compared with tests with fresh pollen in order to make any comparison on viability and germination rates.
Vieira et al. [25], studying pollen grains of accessions of Manihot spp. (cassava), found different results when they compared pollen viability by colorimetric methods and germination ones. They found less viable pollen grains with in vitro tests (germination) than with colorimetric ones. The same is observed here, when we compared viable pollen grain rate (around 68%) with germinated one (22.27%, highest rate), a great difference is faced.
Temperature is a physical factor that affects the germination of pollen grains. Studies of Kakani et al. [26] and Acar and Kakani [27] indicated that both high (above 30˚C) and low (below 20˚C) temperatures can affect pollen germination. Studies of pollen grains of M. emarginata were prosecuted under intermediate temperatures; thereby it is deduced no influence on the results. Furthermore all treatments were processed under the same environmental conditions.
Aguilera and Valenzuela [28] observed that pollen form olive trees cultivated show higher viability rate mainly 24 hours after anther dehiscence, the most probably within a few hours after pollination. This may also explain the low rate of pollen germinability of M. emarginata. As, we used pollen grains of flowers pre-anthesis newly collected and submitted into an artificial way to rest for 72 h, their pollen grains cannot be on their period greatest vigor. This can also be observed with pollen grains of M. emarginata by comparing the viability rates (c. 70%) and germinability (not above 24%) which are very different, the first much higher compared to the second.
This phenomenon, higher pollen germinability after anther dehiscence, is related to the success of fertilization depends not only on pollen production, but also the viability and stigma receptivity. The pollen must have the maximum potential when in contact with the stigma, because stigma as both reproductive cells exhibit responsiveness that lasts only a day or two [29]. A fast decline in pollen viability can greatly reduce effective fertilization and negatively influence the production of fruits and seeds [27].
Lipidic material deposited on pollen wall is the pollenkitt. According to Pacini & Hesse [30], it has many functions, include the adhesion of pollen grain to the stigma surface facilitating the pollen tube penetration.
CPL lectin promoted an increase of pollen germinability, which was expressive by the concentration of 3.0 µg/ ml. Even with low concentration (1.0 µg/ml), the action to pollen germination was noticed, but it was not significant, and similar to control test—growing media without lectin.
This action of CPL lectin to the stimulation of pollen emission of tube is due to its property on cell recognition. When fell on stigma surface, pollen grains are activated and are rehydrated, protruding their tube down on stigma and style towards ovary.
Southworth [11] examined the influence of different lectins (concanavalin A and phytohemagglutinin) in the formation of grains of pollen tubes in Lilium longiflorum Thunb. Since then, other studies have followed this line because lectins have strong influence on the recognition of pollen and on its germination on the stigma, which involves cell signaling processes and genetic incompatibility as shown by Carvalho [31] and Ferrari et al. [32].
Recent studies [33,34] have addressed the action of protein glycoconjugate-endogenous lectins-acting on the polarization of the cytoplasmic membrane in pollen grains of Nicotiana tabacum L. Wan et al. [13] conducted studies of molecular biology and outlined the importance of lectins (mainly lectin RLK-Receptor-Like Kinase). Other authors [35-37] also found that RLK lectins appear to control the development of pollen development by regulating the endothecium, middle lamella and tapetum.
Pollen grains put to grow on medium with lectin act as on a proper stigma surface, rehydrating and producing pollen tube. But it happens in appropriated lectin concentration. Our study showed that under low concentration of CPL lectin pollen grains grow as if they are in a pure medium. So for each plant and each lectin there is an ideal concentration of lectin to be added to a medium.
It is worth to state that in studies with germinability of pollen grains, one should pay attention to growing medium content, mainly to carbohydrates. Because of any lectin is specific to a sugar.
This kind of studies is a window in order to use results of lectin researches to improvement of crops, because lectin can be used in order to increase pollen germination on stigmas and consequently increase productions of fruits and seeds. But more studies are need not only crop by crop, as also to each commercial lectin which may be used for this purpose.
5. Acknowledgements
Authors thank to technicians of Plant Micromorphology Laboratory (LAMIV-UEFS) for assistance; to professors of course of Specialization in Cellular Biology (DCBio/ UEFS) for help during studies; to Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for grant to FAR Santos (Proc. 303557/2010-9).