Dose-Dense Regimen of Cisplatin and Infusional Fluorouracil in Advanced Gastric Cancer—A Single Institution Experience ()
1. Introduction
Adenocarcinoma of the stomach is one of the leading causes of cancer death worldwide. Complete resection is the only curative treatment but approximately two-thirds of patients present with unresectable primary tumor or overt metastatic spread [1]. Furthermore, recurrent disease is noted in up to half of the patients undergoing curative surgery [2-4]. Patients with metastatic disease have a dismal prognosis with a median survival of 5 to 10 months [5-9]. In spite of the fact that multiple chemotherapeutic agents have been studied in advanced gastric cancer (AGC) since the 1970’s, either alone or in combination, the median survival in AGC has not changed significantly.
A very common regimen in AGC used today is a combination of continuous infusion (CI) 5-fluorouracil (5FU) and cisplatin, known as FUP. 5FU and cisplatin have been shown to possess synergistic cytotoxic effects against various human tumors including gastric cancer [10,11].
Cisplatin is assumed to enhance the antitumor effect of 5FU by increasing the availability of the reduced folate necessary for tight binding of fluorodeoxyuridylate, a 5FU metabolite, to deoxythymidylic acid synthase [12, 13]. In addition, several studies and a meta-analysis have shown that 5FU is more effective when administered by CI rather than by bolus injection in several types of cancer including AGC [14-17]. Based on these facts, FUP was evaluated in AGC with good results, and has become one of the most frequently used regimens in this disease [18, 19].
One of the strategies to improve the efficacy of chemotherapeutic regimens is to increase their dose density, i.e., to decrease the interval between treatments. This approach is based on the hypothesis that maximal effectiveness can be achieved by scheduling the chemotherapy intervals to correspond with the period of most rapid tumor growth. Cumulative data, mainly from breast cancer and lymphomas, suggest that dose density can lead to an improved patient outcome without a significant increase in toxicity [20-23].
The dose-dense approach was also evaluated in AGC. In a study by Cascinu et al. [24], 105 patients with AGC were treated with a dose-dense regimen of 5FU, cisplatin, epi-doxorubicin, 6S-leucovorin and glutathione, with the support of stem cell growth factors. The results were encouraging: overall response rate was 62%, the median survival duration was 11 months and the 1- and 2-year survival rates were 42% and 5%, respectively [24].
In the light of the lack of a clear standard regimen in AGC and the favorable results of the dose-dense strategy in other tumors, we decided to adopt this approach in this disease. A dose-dense FUP regimen, given every three instead of four weeks, has already been used in localized and in advanced head and neck cancer [25,26] with a reasonable toxicity profile and we therefore chose this regimen as our standard in AGC. We hereby present our experience using this approach and include an analysis of factors predicting toxicity and benefit from treatment.
2. Patients and Methods
2.1. Eligibility
All patients had histologically confirmed inoperable or metastatic adenocarcinoma of the stomach or gastroesophageal junction (GEJ). Other inclusion criteria were an Eastern Cooperative Oncology Group performance status (PS) ≤3; measurable or evaluable disease; no prior chemotherapy; adequate bone marrow function (defined as leukocyte count >4000 cells/mm3 and platelet count >100,000 cells/mm3); normal liver function (defined as a total bilirubin <1.5 mg/dL, serum aspartate transaminase and alkaline phosphatase <3 times the upper normal limit); and normal renal function (defined as creatinine clearance >60 cc/min). Informed consent was obtained from all patients prior to start of treatment.
Exclusion criteria included a PS of 4, prior chemotherapy, severe co-morbidities, other primary tumors (aside of basal cell carcinoma of the skin and carcinoma in situ of cervix uteri), and inability to sign an informed consent.
2.2. Patient Evaluation
Patient evaluation prior to the initiation of treatment included complete medical history and physical examination, complete blood count (CBC), serum chemistry and creatinine clearance test (CCT), serum tumor markers (carcinoembryonic antigen [CEA] and CA-19.9), and chest and abdominal computerized tomography (CT). During treatment, patients were evaluated for toxicity before each cycle, including full medical history and physical examination in addition to CBC, serum chemistry and CCT. Evaluation of efficacy, including the performance of tumor markers and CT, was done every 2 - 3 courses according to the judgment of the treating physician.
2.3. Treatment
Patients were treated with cisplatin 100 mg/m2 in a 2 hour infusion with adequate prehydration on day 1 followed by CI 5FU 1000 mg/m2/day on days 1 - 5. Antiemetic therapy consisted of 5-hydroxytryptamine-3 receptor antagonists and dexamethasone. The regimen was repeated every 21 days.
2.4. Assessment of Response and Toxicity
Responses were classified according to World Health Organization (WHO) criteria. A complete response (CR) was defined as the disappearance of all measurable lesions with no new lesions for at least 4 weeks. A partial response (PR) was defined as a reduction of at least 50% in the sum of the products of the longest perpendicular diameters of all measurable lesions and the absence of new lesions for at least 4 weeks. Stable disease (SD) was defined as a reduction of less than 50% or an increase of less than 25% in the sum of the products of the perpendicular diameters of all lesions without any evidence of new lesions for at least 4 weeks. Progressive disease (PD) was defined as an increase of greater than 25% in tumor size or the appearance of new lesions. Toxicities were graded according to the CTC-NCI version 2.
2.5. Statistics
Overall survival (OS) was calculated from the start of treatment to death or the last date the patient was known to be alive. Progression-free survival (PFS) was calculated from the start of treatment to the date of documented progression or the last date the patient was known to be progression-free. The product limit estimated method (Kaplan-Meier) was used to estimate OS and PFS intervals. In order to compare response to treatment, OS and PFS according to various clinicopathological factors, Chi-Square and log-rank test were used. A pvalue of less or equal to 0.05 were considered statistically significant.
3. Results
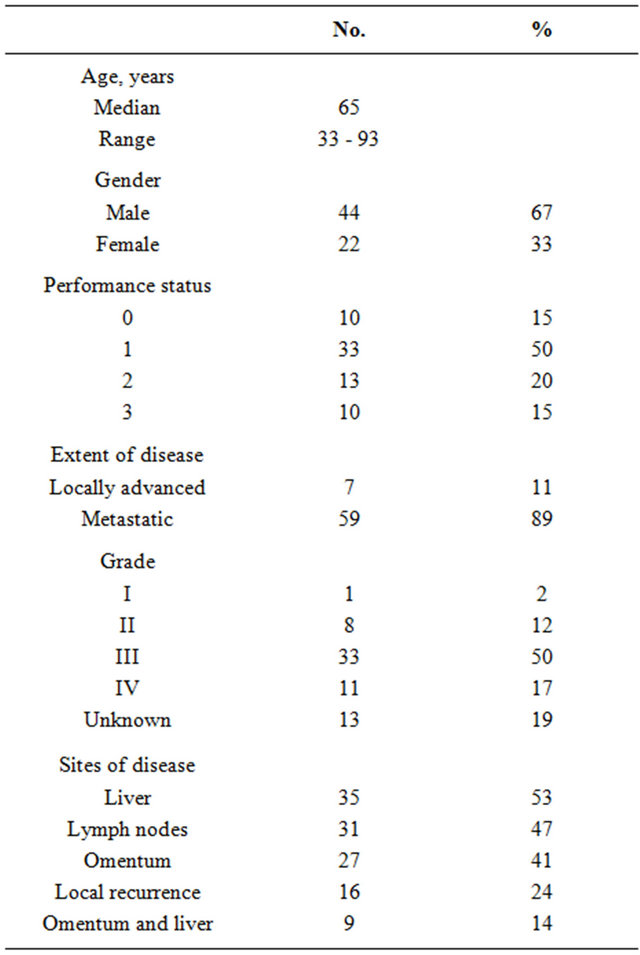
Sixty-six patients were enrolled in the study. Patient characteristics are summarized in Table 1. The median age was 65 years (range: 33 - 93) and the majority of patients were male (67%). Sixty-five percent of the patients had a good PS (0 - 1). The majority of patients (89%) had metastatic disease, which almost invariably involved the liver, omentum or both.
Table 1. Patient characteristics (n = 66).
3.1. Safety and Dose Intensity
All 66 patients received at least one cycle of chemotherapy and were assessable for toxicity and dose-intensity (DI) analysis. The median number of treatment cycles administered was 3 (range, 1 - 6). The median DI of 5FU and cisplatin was 90% (range, 49% - 100%) and 93% (range, 58% - 100%) of the planned dose, respectively.
The toxicities observed in the study population are listed in Table 2. Chemotherapy was associated with significant toxicity. Treatment was discontinued due to toxicity in 12 patients (18%) and due to patient request in 2 others (3%). There were 4 toxic deaths (6%): 2 patients died from neutropenic sepsis and 2 from diarrhea. The most common overall toxicities were fatigue, leucopenia and mucositis, while the most common grade ≥3 toxicities were fatigue, nausea/vomiting and leucopenia, noted in 42%, 30% and 12% of the patients, respectively.
The two variables which were found to predict toxicity were PS at the start of treatment and age. Poor PS (≥2) was associated with an increased risk for grade ≥3 hematological toxicity including leucopenia (p = 0.03), anemia (p = 0.003) and thrombocytopenia (p = 0.013), as well as for severe fatigue (p = 0.028). Age ≥65 was associated with increased grade ≥3 vomiting (p = 0.04), anemia (p = 0.05) and leucopenia (p = 0.05).
3.2. Response to Treatment
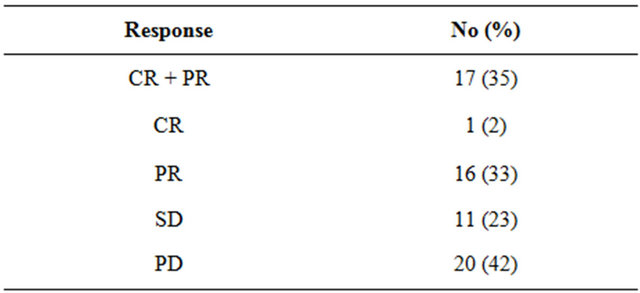
Forty-eight of the 66 patients (73%) were evaluable for response. Sixteen patients (24%) were unevaluable due to early stoppage of treatment because of toxicity or rapid clinical deterioration and 2 (3%) were lost to follow-up before evaluation. Tumor responses to chemotherapy are summarized in Table 3. Response was noted in 17 of the 48 patients, including one CR (2%), with an overall objective response rate of 35%. Eleven patients (23%) had SD for at least one month as their best response. The rate of disease control (CR + PR + SD) was therefore 58%.
We examined the relationship between various clinicopathological factors, including PS, stage, grade, age and gender, and the response to FUP therapy. On univariate analysis, response to treatment was found to correlate with PS (p = 0.0001) and the presence of liver metastases (p = 0.002). However, in multivariate analysis only the presence or absence of liver metastases retained statistical significance (p = 0.006). Liver metastases showed a higher response rate (65%) than omental (38%) or lymph node metastases (45%).
Table 2. Incidence of toxicity.
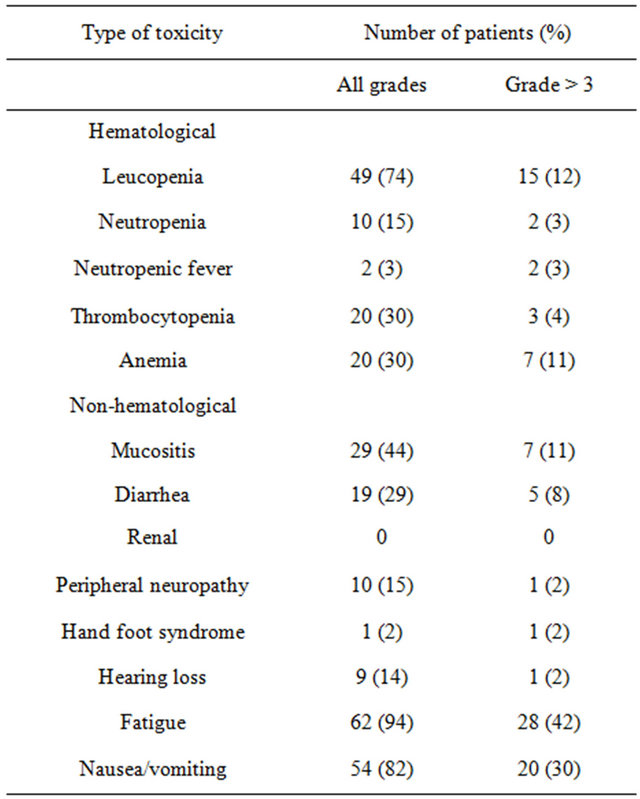
Table 3. Overall objective response rate.
3.3. Survival
The median OS of the entire group was 5.9 months (range 1 - 29) and the median PFS was 4.3 months (range 0 - 26). On univariate and multivariate analyses, factors that correlated with poor survival were presence of liver metastases (HR = 1.7, p = 0.0546) and poor PS (HR = 2.4, p = 0.0001). PS was also a prognostic factor for PFS (HR = 1.88, p = 0.0001).
4. Discussion
The current study summarizes the results of an attempt that we have made to adopt the dose-density concept into the treatment of AGC. While the data presented were not produced within a framework of a prospective study, they still provide the only piece of information available to date on the potential impact of this approach on the performance of a very common regimen, FUP, in AGC. In the only prior use of a dose-dense regimen in AGC, Cascinu et al. evaluated an investigational combination of 5FU, cisplatin, epidoxorubicin, 6S-leucovorin and glutathione [24].
So, does a dose-dense FUP regimen, as used in our study, represent a potentially improved FUP regimen? It appears that the answer is a resounding no; response rate of 35%, median PFS of 4.3 months and median OS of 5.9 months, as noted in our study, are at best equal to those obtained by standard FUP regimens in AGC. For example, in two phase II trials, standard FUP yielded response rates of 41% and 43% and a median survival of 9 months [18,19]. Data from phase III randomized trials is in line with the phase II data. In a study from Korea, where FUP was compared with 5FU alone or a combination of 5FU, doxorubicin and mitomycin C (FAM), the response rate in the FUP arm was 51% and the median survival was 9 months [27].
In another phase III trial, by the European Organization for Research and Treatment of Cancer (EORTC), where FUP was compared with combinations of epirubicin, 5FU and leucovorin (ELF) or 5FU, doxorubicin and methotrexate (FAMTX), the response rate of FUP was 20% and the median survival was 7.2 months [28]. Similar results were noted in the control arm of the V325 trial, evaluating the addition of Docetaxel to FUP [29].
The toxicity profile of the dose-dense FUP also did not seem to compare favorably with standard FUP regimens. In fact, the incidence and severity of toxic events in the dose-dense FUP were higher than those observed with standard FUP [18,19]. Most notably, the rate of treatment-related deaths recorded in our study (6%) is unreasonably high.
The lack of enhanced efficacy and the higher toxicity of the dose-dense FUP regimen may be related to two main possible causes: an innate inferiority of the modified regimen or substantial differences between our patient population and those treated in the studies using the standard regimen. Our study population could indeed be considered a poor-risk group, especially when compared with the patients treated in the non-randomized phase II trials: 52% of our patients were 65 years or older, 15% had PS = 3, 67% had poorly differentiated tumors, and 53% had liver metastases. It is unclear, however, if the disappointing results of the dose-dense FUP regimen merely represent the difference between an unselected patient population treated in daily practice to those being treated within clinical trials, or an inferior treatment approach, or both. To put our results in perspective, a comparison of the main patient characteristics and toxicities, as well as efficacy end-points, between our study and several studies using standard FUP regimens, are summarized in Table 4.
In this study we also evaluated prognostic factors predicting response to treatment and survival. Presence of liver metastases was the only factor that correlated with response to treatment. The finding that liver metastases respond better to chemotherapy is in concordance with the results reported by Kondo et al. [30]. However, in two other studies, a lower response rate for liver metastases compared with the primary lesion or lymph nodes was reported [31,32]. Similar to other studies, we also did not find a significant correlation between response rate and PS [33-35].
Prognostic factor analysis for patient outcome identified the presence of liver metastases and poor PS to adversely affect survival. These results are comparable with prior reports [36-38]. Other widely accepted prognostic factors, such as tumor grade, presence of bone metastases, peritoneal metastases and ascites, did not show a prognostic impact in the current study [39,40]. Of note is the fact that liver metastases respond better to chemotherapy but are at the same time associated with dismal prognosis, emphasizing the aggressive biology of AGC characterized by significant yet transitory chemosensitivity.
5. Conclusion
The dose-dense FUP regimen, as used in our study, failed
Table 4. Comparison of the current study with studies using standard FUP regimens.

to present a potential progress in the treatment of AGC. In the light of the obvious limitations of our retrospective data, it is currently unclear whether the dose-dense strategy has indeed no role in this disease. In any case, we find no reason at the present to replace the standard fourweekly FUP regimen, particularly with the increased quality of life afforded by the longer interval between treatments.
Abbreviations
CR: complete response;
PR: partial response;
SD: stable disease;
PD: progressive disease;
PS: performance status;
PD: pathology-poorly differentiated pathology;
PFS: progression free survival;
OS: overall survival.
NOTES
#Corresponding author.