Exposure to Hyaluronan and Radon-Containing Water during the Treatment of Periodontal Pockets ()
1. Introduction
In parallel, risk factor studies have illuminated the broader implications of inflammatory periodontal diseases, emphasizing a 1.6-fold higher prevalence of chronic somatic diseases in young individuals compared to control groups. Among these, gastrointestinal tract disorders emerge as primary somatic pathologies, highlighting the systemic impact of periodontal health. Noteworthy is the observation that young individuals exhibit a 2.5 to 2.6 times higher susceptibility to respiratory ailments, coupled with a significant hereditary predisposition to periodontal disease. Furthermore, lifestyle factors, such as smoking and dietary preferences, contribute to an increased incidence of inflammatory periodontal diseases [1] .
In selecting Tskaltubo water as the medium for this research, several compelling factors underpin this strategic choice. Tskaltubo water is renowned for its unique mineral composition, featuring a harmonious blend of trace elements and compounds that have demonstrated therapeutic properties in various medical contexts. The high mineral content of Tskaltubo water, including its radon concentration, is known to exhibit potential health benefits, making it an intriguing candidate for investigation [1] .
Tskaltubo, a region celebrated for its natural thermal springs, has been historically associated with spa and wellness traditions, attesting to the therapeutic qualities of its water. The mineral-rich composition of Tskaltubo water is believed to contribute to its hormetic effect, a phenomenon wherein low doses of otherwise harmful agents confer beneficial effects on biological systems. This property aligns with the research objective of exploring the hormetic effects of radon in conjunction with hyaluronic acid [2] [3] .
Furthermore, the radon content in Tskaltubo water falls within a range considered normal and has been recognized for its potential health-promoting attributes. The selection of Tskaltubo water as the medium allows for a controlled environment to investigate the specific effects of radon on hyaluronic acid and its applications in dental practice [4] .
Overall, the choice of Tskaltubo water as the medium is rooted in its rich mineral composition, historical association with therapeutic benefits, and the potential hormetic effects of its radon content, all of which contribute to a robust and relevant research context for exploring the interactions between hyaluronic acid and radon in the dental field [1] [5] [6] [7] [8] .
This refined introduction sets the stage by integrating a more extensive review of literature related to HA, radon water, and their applications in dental practice, providing a comprehensive context for the subsequent discussion [9] [10] [11] .
2. Mechanism of Action of Hyaluronic Acid
Hyaluronic acid is a commonly used preparation, and HYADENT BG (Image 1) is a specifically designed non-animal hyaluronic acid-based treatment for optimal regeneration in dental practice. It facilitates the rapid healing and restoration of wounds and damaged tissues, expediting the regeneration process. Studies have assessed the efficacy of injecting hyaluronic acid into periodontal pockets, leading to its frequent use in dentistry as a unique remedy. Each 1 ml of Hyadent BG contains 2.0 mg of hyaluronic acid (equivalent to 16.0 mg), 6.9 mg of sodium chloride, and 1.0 ml of water for injection [6] [12] .
The primary objective of the study is to investigate and understand the hormetic effect of hyaluronic acid and radon water. This investigation aims to provide a more precise determination of the potential effectiveness of a specific intervention and the post-procedural prognosis for individual patients. By exploring their combined effects, clinical characteristics, and neuro-humoral processes related to inflammatory periodontal diseases (periodontitis), we can establish an effective scheme for post-procedural patient monitoring.
This comprehensive review will enable patients to achieve a quicker and cost-effective resolution of inflammatory periodontitis. Simultaneously, it will contribute to the growth of medical tourism, enhancing the overall treatment experience. The use of hyaluronic acid, coupled with the hormetic effect of radon in the water and the presence of micro and macro elements, distinguishes the water as unique, facilitating the treatment of periodontitis (Image 2) and ultimately leading to the healing and disappearance of oral cavity issues [8] .

Image 1. Hyadent BG and adminstartion demonstration.
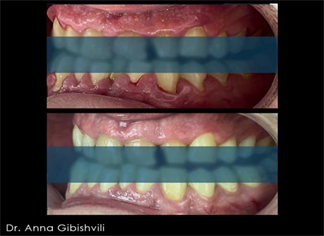
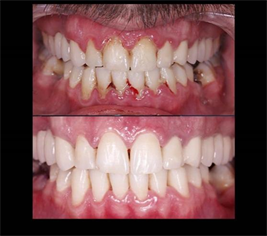
Image 2. Stage 1 Periodontitis, before and after treatment.
The specific issue mentioned could not be found in the literature that was searched. As a result, the outcomes of the research have the potential to serve as both scientific news and the foundation for practical recommendations.
Selection Rationale for Hyadent BG: Hyadent BG was chosen as the hyaluronic acid gel for this study due to its unique formulation and proven efficacy in promoting optimal regeneration in dental applications. The selection was based on several key factors:
1) Non-Animal Sourced Hyaluronic Acid: Hyadent BG is derived from non-animal sources, ensuring ethical sourcing and reducing the risk of potential allergic reactions. This characteristic aligns with contemporary preferences for ethical and biocompatible dental interventions.
2) Regenerative Properties: The formulation of Hyadent BG has been specifically designed to facilitate rapid healing and tissue restoration. Its regenerative properties make it particularly suitable for addressing periodontal issues, contributing to the overall success of the study’s objectives.
3) Clinical Evidence: Previous clinical studies and practical applications have demonstrated the effectiveness of Hyadent BG in promoting tissue regeneration in dental procedures. The existing body of evidence supports its inclusion in this study as a reliable and clinically proven hyaluronic acid gel.
Administration Details: The administration of Hyadent BG in the study followed a meticulous protocol to ensure standardized and consistent application. The gel was injected into periodontal pockets using a three-step treatment method:
1) Preparation: The patients underwent thorough oral hygiene and individual hygienic adjustments before the administration of Hyadent BG. This step aimed to create an optimal environment for the subsequent application of the hyaluronic acid gel.
2) Anti-Inflammatory Therapy: Following the oral hygiene phase, patients received anti-inflammatory therapy based on their clinical condition. This preparatory step was crucial to address any existing inflammation and optimize the conditions for the subsequent application of Hyadent BG.
3) Injection Protocol: The first group of patients received injections of 1 ml of Hyadent BG into the periodontal pockets. The injections were administered using a specific needle with precise angles and depths, ensuring proper tissue penetration (Image 3). The injection process followed a three-step treatment
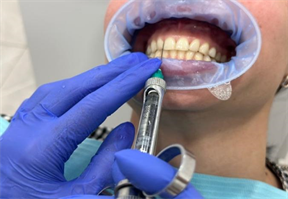
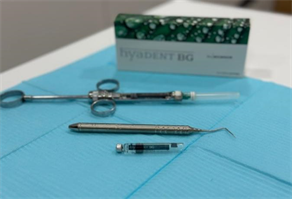
Image 3. Materials used and injection method.
method, contributing to the controlled and effective delivery of the hyaluronic acid gel.
By selecting Hyadent BG and adhering to a detailed administration protocol, the study aimed to maximize the therapeutic potential of hyaluronic acid in addressing periodontal issues and contributing valuable insights to the field of dental research.
3. Material and Methods
In the research method, low-molecular-weight HA was utilized, and Tskaltubo water served as the medium. The radon content in the water was measured to be 37 bq, which is within the normal range, whereas building materials typically contain 123 bq of radon. Consequently, the water stands out due to its hormetic effect (R-Ho), which triggers the activation of nitric oxide (NO) and subsequently enhances peripheral microcirculation and central hemodynamics. NO can be regarded as a homeostatic modulator. Radon also plays a crucial role in regulating sodium (Na+), potassium (K+), and calcium (Ca2+) ion fluctuations. It acts as a suppressant of specific autoimmune diseases while activating the body’s non-specific immune system. Radon possesses several essential properties, including pain relief, reparative regeneration, immunomodulation, stress induction, and antibacterial and antiviral effects. Moreover, it regulates inflammatory processes and acts as an antioxidant by removing reactive oxygen species (ROS) from tissues. Hence, small doses of radon present in tap water can aid in stabilizing the granulation tissue matrix.
HA demonstrates excellent physiochemical and biological properties, making it widely recognized and utilized in various medical fields such as orthopedics, dermatology, and ophthalmology, particularly in the treatment of inflammatory processes. When HA is dissolved in water, its hygroscopic properties come into play, as it interacts with hydrogen molecules, carboxyl groups, and N-acetyl groups. This unique property allows HA to maintain its rigidity and conformational stability. Remarkably, one gram of HA can bind approximately 5 - 6 liters of water. HA also possesses physical properties that enable it to fill empty spaces within tissues, absorb shocks, and selectively bind proteins [3] [13] . As evident from the literature, low molecular weight HA exhibits antibacterial effects, particularly against strains such as aggregatibacter actinomycetemcomitans, Prevotella oris, and Staphylococcus aureus, which are commonly found in gingival lesions and various periodontal wounds. With these remarkable properties, HA can reduce the occurrence of postoperative infections and facilitate planned regeneration following surgery. Furthermore, its biocompatibility and involvement in post-surgical processes, such as periodontal tissue healing and regeneration, further highlight its beneficial attributes [5] [6] [7] . A total of 30 patients diagnosed with grade A slowly progressing periodontitis, without severe somatic pathology, participated in the study.
Patient Selection and Diagnostic Criteria: The inclusion of participants in this study was based on a meticulous diagnosis of grade A slowly progressing periodontitis, involving a comprehensive evaluation of clinical and radiographic parameters. The criteria for diagnosing grade A periodontitis encompassed probing depth measurements, clinical attachment level assessments, bleeding on probing, and radiographic evidence of bone loss. Patients demonstrating characteristics indicative of mild and slowly progressing periodontal disease were considered eligible for participation. Specifically, individuals with probing depths between 3 to 4 millimeters, minimal clinical attachment loss, absence of deep periodontal pockets, and no evidence of advanced bone loss were identified as fitting the criteria for grade A periodontitis.
Assessment of Somatic Pathology: To ascertain the absence of severe somatic pathology, a thorough examination of each participant’s medical history was conducted. This assessment involved a review of existing medical records, discussions with other healthcare providers when applicable, and consideration of chronic diseases or conditions that might significantly impact the patient’s overall health. Patients with systemic conditions, such as uncontrolled diabetes or cardiovascular diseases, that could potentially confound the study outcomes were excluded.
In addition to the medical history review, participants underwent a general health assessment, including physical examinations and laboratory tests as needed, to ensure the absence of severe somatic pathology. Non-smoking status was also confirmed during this process, contributing to the overall health profile of the study participants.
By adhering to these criteria, the study aimed to create a homogeneous group of participants with grade A slowly progressing periodontitis, minimizing the influence of confounding factors related to systemic health issues. This approach enhances the internal validity of the study, allowing for a more accurate assessment of the effects of interventions such as hyaluronic acid injections and radon-containing water inhalation on periodontal health [1] [14] [15] [16] .
These patients were divided into two equal groups consisting of 15 individuals each: a control group and a main group on which the study was conducted. Both male and female participants who were non-smokers were included, and there were no notable differences in the development of periodontal inflammation between them. Several days prior to the examination, the patients were informed about the study conditions and were given specific instructions to consult the doctor. They received both written and oral information during the doctor’s interview and provided their consent accordingly [5] [11] .
The dental interview conducted by the doctor before commencing the comprehensive study included a survey, collection of medical history, examination of the patient’s oral cavity, assessment of the condition of the oral mucosa and periodontal tissues, and filling out the periodontological map. The periodontal index and the deepest clinical attachment level (KMD) were determined. In cases where interdental spaces exhibited 1 - 2 mm depths and the coronal space of the root showed less than 15% involvement, an X-ray examination was usually suggested to further evaluate the extent of bone tissue damage and assess the nature, stage, and severity of the pathological condition. A description of the obtained photographs or a photo protocol was prepared [7] [11] [17] .
Both groups of patients underwent a study that involved oral hygiene and individual hygienic adjustments or corrections. Subsequently, anti-inflammatory therapy was prescribed based on the patient’s clinical condition [18] [19] [20] .
Additionally, the first group of patients received injections of 1 ml of Hyadent BG (Image 4), which contained hyaluronic acid (2.0 mg), equivalent hyaluronic acid (16.0 mg), sodium chloride (6.9 mg), and injection water (1.0 ml) into the periodontal pockets of Ha Gel. Hyadent BG is manufactured by the German company BioScience GmbH (BS091). The hyaluronic acid gel consists of a mixture of cross-linked (1.6%) and natural (0.2%) hyaluronic acid in 2 × 1.2 ml cylindrical ampoules [12] [21] [22] .
Exactly one week after the professional oral hygiene treatment, the drug was administered according to the protocol, which included a three-step treatment method. Revident or Ha was injected with a needle of 0.1 ml at an inclination of 300 to 450 angles into the dental-gingival groove along its fold (Image 5), five times, at a depth of mm. Retrograde movements of the needle were employed to ensure proper tissue penetration. The protocol also involved inhalation with Tskaltubo water. A comparison was made between patients who lived in Tskaltubo or Kutaisi and underwent inhalation of Tskaltubo water, and patients who received only Ha injections (Image 5) to evaluate the combined effect of Ha and water inhalation from a water tube [9] [10] [11] .
The treatment protocol further included inhalation with water from a radon-containing water tube. Radon-containing water was used to address
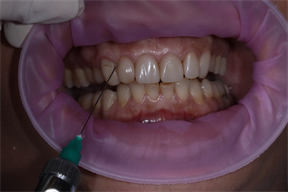
Image 4. Injection of Hyadent BG.
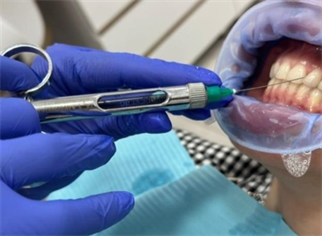
Image 5. Hyaluronic acid injection in the interdental periodontal pocket.
inaccessible areas where subgingival calculus could not be removed using piezoelectric devices or hand curettes, or where the calculus remained after polishing procedures. Radon water assisted in breaking down the calculus and biofilm matrices, facilitating their easy removal from the tooth’s surface and surrounding tissues [5] [9] [21] [22] [23] .
A sensor operating at a signal frequency of 25 MHz was positioned on the gingival border and within the periodontal pocket groove of all maxillary and mandibular teeth. The blood flow condition was assessed by analyzing the Doppler signal through the Minimax Doppler program [9] [24] . For the quantitative analysis, we calculated the mean and the standard deviation. Group comparisons were conducted using the independent t-test, while the paired t-test and ANOVA were used to assess changes before and after treatment. Qualitative indicators were compared between groups using Fisher’s exact test, and pre-and post-treatment comparisons were performed using the Wilcoxon test. Mathematical analysis was performed using the IBM SPSS v22.0 software package to ensure statistical accuracy.
As an additional examination method, ultrasound dopplerography of the periodontal blood vessels was conducted. This study of the periodontal microvasculature was carried out using a domestically produced specialized portable ultrasound device called “Minimax-Doppler-K”. The device was used to record the microcirculation parameters of the periodontal tissues.
Ethical considerations were duly addressed in the study. The questionnaire used in the research fully adhered to the principles outlined in the Helsinki Declaration of the World Medical Association and complied with the national legislation of Georgia concerning the protection of patient rights and the conduct of medical research [15] .
4. The Purpose of the Study
Study of the effectiveness of the use of the drug HA in the complex treatment of periodontal diseases.
Hyadent BG “BS091”.
• Cross-linked hyaluronic acid;
• Concentration 1.6% Cl + 0.2% NCL;
• Without endotoxins of synthetic origin;
• Elimination period: 4 - 6 weeks;
• Packaging: 2 capsules, each 1.2 ml.
(HYADENT BG BS091 low-molecular hyaluronic acid. It consists of a mixture of cross-linked (1.6%) and natural (0.2%) hyaluronic acid. HYADENT BG is produced by the German company BioScience GmbH—exclusive representative in Georgia—Inverto Medical LLC [10] .
5. Results and Discussion
The combined functions of Tskaltubo water and HA contribute to the preservation of tissue’s structural and homeostatic integrity. These substances possess specific characteristics, including high viscosity, the ability to bind water and proteins, and the formation of proteoglycan aggregates, which enable them to fulfill the trophic, barrier, and plastic functions of connective tissue [20] . Based on these properties, we utilized the effects of both Tskaltubo water and HA in the treatment of periodontal grade A, which is characterized by a significant buildup of biofilm on the tooth surface with minimal tissue destruction [1] .
Thus, it can be stated that both Tskaltubo water and HA have an impact on both acute and 23 chronic processes, leading to the modulation of inflammatory processes [11] [20] [21] . Notably, both substances strengthen the effects of metalloproteinases, effectively preventing tissue destruction, as observed with water from the tub. This water blocks the arachidonic acid cascade at the initial stage of inflammation, while the synthesis of prostaglandins, which are vital protective factors, remains undisturbed [8] . This is manifested through the activation of inhibitors. Consequently, a similar effect is achieved by slowing down the production of cytokines, such as TNF-α, an anti-inflammatory cytokine. HA aids in maintaining tissues damaged by inflammation, and it has been reported in the literature that HA exhibits a bacteriostatic effect against microorganisms found in periodontal tissues, including A. actionomyecetemcomitans and Prevotella intermedia, among others (Becker et al.). Thus, HA provides biological protection after professional hygiene, in which the radon-containing water of Tskaltubo also participates. The joint action of HA and Tskaltubo’s radon-containing water is essential during the surgical treatment of periodontal pathology. Together, they activate fibroblasts, which produce collagen fibers [5] [21] and stimulate the synthesis of cytokines by fibroblasts, keratinocytes, cementoblasts, and osteoblasts. Consequently, they promote the synthesis of endogenous HA by endothelial cells. Both HA and Tskaltubo’s radon-protected water contribute to cell migration, proliferation, and differentiation processes [6] [12] .
During our clinical examination of periodontal tissues in 129 patients aged 20 to 39 years, we detected inflammatory periodontal diseases in 95.3% of the patients, with 46.5% classified as mild periodontal grade A. Therefore, our studies were conducted on these patients [9] [10] .
As reported in the literature, the number of patients with inflammatory periodontal diseases continues to increase each year. Currently, inflammatory periodontal diseases rank second in terms of frequency and prevalence among all dental diseases. Extensive epidemiological studies on periodontal diseases have significantly changed the general understanding of their etiology and pathogenesis, leading to new priorities in diagnosis and treatment. Most cases of periodontitis have a chronic inflammatory component, necessitating the use of antiseptic, anti-inflammatory, and immunostimulating agents as crucial components of comprehensive pathogenetic treatment. However, it is important to note that the use of anti-inflammatory and antibacterial drugs can reduce the body’s immunobiological reactivity, leading to allergic reactions and other undesired side effects. Presently, a majority of patients with periodontal pathology also have allergies or other associated somatic conditions. Consequently, the options for treating inflammatory periodontal diseases are severely limited in such cases, emphasizing the need to explore alternative treatment approaches. Therefore, we aimed to investigate alternative treatment methods utilizing Tskaltubo water and hyaluronic acid and demonstrate their combined effects [2] [5] [25] .
Manufacturer: BioScience GmbH. www.bio-science.org Name of the drug: HyaDENT BG.
During the clinical examination of the patients, several observations were made. Firstly, exposure to both HA and the radon-containing water from the water tube resulted in a reduction of gingival swelling and hyperemia. Additionally, there was an improvement in the PMA (Plaque Control Record) and BOP (Bleeding on Probing) indices, as well as an enhancement in blood flow velocity as indicated by periodontal ultrasound dopplerography (Image 6). Notably, the first group of patients exhibited higher levels of the examined parameters compared to the second group [2] [4] [14] .
The survey further revealed that the patients reported a noticeable improvement in their gum condition, accompanied by a reduction in pain, and eventual disappearance of pain during the subsequent period. In both groups, significant changes were observed in hygiene indices (OHI-s), PMA, bleeding index (BOP) [17] [20] [26] , and KPI (Keratinized Tissue Probing Depth) index (Graph 1) based on the results of intragroup analysis (Table 1).
In the first group, the oral hygiene status indicators (OHI-s) were 2.7 points before treatment. After hyaluronan treatment, the score improved to 1.55 points, indicating an unsatisfactory oral hygiene level. However, 10 days after treatment, the rating improved to 1.1, indicating partially satisfactory oral hygiene. The combined effects of both treatments were rated at 0.9, indicating that the hygiene level was within satisfactory limits. The evaluation following exposure to both treatments resulted in a score of 0.6, indicating a good level of oral hygiene [17] [21] .

Image 6. The data obtained from the Doppler ultrasound during the research were assessed both qualitatively and by considering the quantitative characteristics.
![]()
Table 1. The average indicators of periodontal characteristics were evaluated 10 days after treatment.
![]()
Graph 1. Represents the evaluation of the KPA (Keratinized Tissue Probing Depth) index based on the action of HA acid and HA + radon-containing water.
Significant differences were observed in the inter-group analysis of the second group. This suggests a more pronounced clinical effect, particularly with HA implantation. Furthermore, the observed results indicate a faster outcome with the introduction of HA and exposure to radon-containing water as part of the comprehensive treatment approach for periodontal inflammatory diseases.
According to the analysis of the PMA (Plaque Control Record) index (Graph 2), it was observed that the reduction in the PMA index for patients in group I was 36.22. After HA treatment, it decreased to 11.6, and following exposure to both HA and radon-containing water, it further decreased to 0.501.
![]()
Graph 2. Depicts the evaluation of changes in the PMA (Plaque Control Record) index of the oral cavity based on the action of HA acid and HA + radon-containing water.
Notable changes were also observed in the OHI (Oral Hygiene Index) indices (Graph 3). Before treatment, the OHI score was 2.7, which decreased to 1.55 after HA treatment in group I. In the second group, the OHI score was reduced to 1.1. After 10 days, the OHI score further improved to 0.9 in both groups.
The data obtained from the study indicate that the use of HA acid creates favorable conditions that contribute to an improvement in the oral hygiene level. Moreover, when both HA acid and radon-containing water are used together, an excellent result is achieved within 10 days. This result is close to the normal range and, in some cases, appears to be normal. It is important to note that such a study is not commonly found in the literature. Our primary objective was to demonstrate how the hygienic index, PMA index, KPI (Keratinized Tissue Probing Depth), and periodontitis tissue can be effectively treated in a painless manner and with long-lasting effects within a short period of time.
The analysis of the blood flow velocity curve involves both qualitative and quantitative assessments. The qualitative characteristic of the Doppler curve typically varies depending on the type and caliber of the blood vessels. Mixed blood flow is characterized by a wave-like pattern in the color spectrum without sharp peaks [26] .
The average linear velocity of blood flow (Vam) serves as a crucial diagnostic criterion for microcirculation disorders in periodontal tissues. It enables the determination of the degree of disorder and the severity of the pathological progression in the periodontium [21] [23] .
![]()
Graph 3. Illustrates the evaluation of changes in oral hygiene level based on the action of OHI-S HA acid and HA + radon-containing water.
The initial values of the Vam (average linear velocity of blood flow) indicator in the 1st and 2nd groups were practically at the same level, measuring 0.53 cm/s and 0.52 cm/s, respectively. However, one week after treatment, the indicator increased to 0.78 cm/s in group 1 and 0.61 cm/s in group 2.
After one month of treatment, the first group maintained a consistently higher-than-average value of 0.65 for the linear velocity of blood flow. In contrast, the second group, which underwent exposure to HA and radon-containing water, maintained a stable high value of 0.75.
6. Conclusions
1) Evaluation of changes in oral hygiene level (OHI-S) indicates that the combined action of HA acid and radon-containing water results in a faster healing effect compared to HA treatment alone.
2) Evaluation of changes in the oral PMA (Plaque Control Record) index level shows that the combined action of HA acid and radon-containing water leads to a faster healing effect compared to HA treatment alone.
3) Evaluation of changes in the oral PAM (Periodontal Attachment Level) index level reveals that the combined action of HA acid and radon-containing water results in a faster healing effect of the oral cavity compared to HA treatment alone.
4) After one month of treatment, the linear velocity of blood flow in the first group remains consistently higher than average, measuring 0.65. In the second group, where exposure to HA and radon-containing water occurred, the linear velocity of blood flow remains stably high at 0.75.