1. Introduction
Cavitation in plants is defined as void formation in sap, which leads to blockage of water transport [1] . Zimmermann put forward the hypothesis of “air seeding” to explain cavitation formation [2] . Lewis observed the process of gradually extending of bubbles in hyalocysts of branch leaves of Sphagnum [3] . Cavitation can be detected by acoustic emissions [4] [5] . Bordered pit membranes play a crucial role in drought-induced embolism spread via the process of air-seeding [6] . Then, functional implications of intervessel connections are still not well understood [7] [8] [9] .
Ponomarenko et al. [10] distinguished two types of optical events. Following their work, Shen [11] analyzed two types of cavitation: First type is the “nucleation” events, which may be caused by the growth of pre-existent air bubbles; the other is air seeding, here defined as an air bubble is sucked into a conduit from an already gas-filled conduit. The two types of cavitation include three ways. For the first way, at higher water potential along with the drop of water potential, an air bubble will swell gradually to form a long-shape bubble, and extend continually. For the second way, a seed becomes larger with the drop of xylem pressure till it reaches a threshold, causing the bubble to blow up to form a bubble in long shape. It will extend further as xylem pressure decreases continually. When xylem pressure  is lower than
is lower than  (
(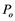 is atmospheric pressure), as soon as an air seed is sucked into a conduit it will explode immediately and the conduit will be full of the seed air instantly, leaving little water at the end (or two ends) of the conduit [12] [13] . This is the third way. The second and third ways all include explosion event.
is atmospheric pressure), as soon as an air seed is sucked into a conduit it will explode immediately and the conduit will be full of the seed air instantly, leaving little water at the end (or two ends) of the conduit [12] [13] . This is the third way. The second and third ways all include explosion event.
In reality the two types of cavitation in conduits only involve two processes: the gradual expansion and elongation, and the explosion event.
The explosion event of air bubble can only take place in intact conduits at water tension; when an air bubble breaks, acoustic (or ultrasound) emission is triggered, and, simultaneously, with the sap retreating, the air in it, diffuses at high speed, forming blurry boundary of water/air. The action of the sap in the conduit is like to the soap film on a metal ring, where as soon as the soap film breaks, the soap liquid retreats to the metal ring at once.
In the experiment of Ponomarenko et al. [10] the ultrasound emission was triggered by bubble nucleation, and the embolism development was the abrupt appearance of bubbles and their intermittent development from several nuclei. In the experiment of Mayr et al. [14] the acoustic emission and the movement of the air at high speeds took place at the same time and the shift of menisci could hardly be followed. Here, we suggest that above phenomena should be caused by bubble explosion in tension water in the intact conduits.
Cavitation phenomenon has been observed using several different types of instruments [7] [8] [15] - [21] . Then, conventional microscopy, e.g. light microscopy and cryo-scanning electron microscopy, are usually material destructive. On the other hand, although Magnetic Resonance Imaging (MRI) can be used to monitor processes of cavitation and embolism repair directly and dynamically in real time, it requires specimen small enough to fit the sample chamber; In addition, its spatial resolution of about 10 - 100 μm is not enough to distinguish between small conduits.
XPCM is non-destructive, real-time dynamic micro-imaging technique. As there is no need for sample to be transparent, XPCM provides a feasible technique to investigate the cavitation process in intact conduit of plants.
Choat et al. [22] have observed the spread of embolism during dehydration by synchrotron x-ray micro tomography, but no detail of instant cavitation process was reported.
In this study, we employed XPCM to record the dehydrating processes in leaves of corn and rice, and captured the process of air diffusing at high speed in intact vessels.
Before XPCM experiment, several preliminary experiments were done. Based on the results, cavitation processes of the samples could be captured more easily by acquiring consecutive XPCM images.
At last we suggest that for some whole plant the embolism formation is caused by explosion event of cavitation and for embolism spread the explosion event of air seed may be the most important.
2. Materials and Methods
2.1. Selecting Materials
Tree stems contain more vessels and cavitation event takes place frequently. When a stem was used in our XPCM experiment, since stem was thick, the information of the sap movement in it overlapped. Therefore, plant leaves were selected as materials. Among them, what the vascular bundle are arranged neatly became the first choice. Then, the leaves of rice and corn were selected as materials.
2.2. Preliminary Experiments
1) Paraffin sections of the leaves of different species were observed under light microscope to know their structure.
After selecting the type of plant leaves, for each special, we should know the occurrence area of cavitation event in a leaf. Thus, Paraffin sections of the leaves were observed under light microscope to provide the structure of the leaves.
2) Determining the occurrence time of cavitation event by ultrasonic emission
In x-ray experiment, a large number of imaging frames could be gotten during the experimental period. It was very hard for us to select the useful frames, which showed the air moving instantly. We have known that for a cavitation event, ultrasound emission and air diffusing at high speed occur, simultaneously, thus, testing ultrasound emission could help us to determine the time range of cavitation events.
3) Determination of water potential of leaves by Small Fluid Flow Method
According to our theory, an exploding event can only take place at water tension. If we wish to get the first way of cavitation of a leaf, it should be put in water for a period of time, letting it be saturated with water. We wish to find out the two processes of a leaf in XPCM experiment and to know the moisture state corresponding to each of them. Thus, the water potentials of leaves were determined by Small Fluid Flow Method.
2.3. Synchrotron X-Ray Phase Contrast Microscopy
Real-time observation of cavitation event of leaves using XPCM has been carried out at X-ray Imaging and Biomedical Application Beamline (BL13W1) at Shanghai Synchrotron Radiation Facility (SSRF) (Figure 1). The leaves of corn and rice are relatively thin and they have well-developed conduits and regular vascular bundles in the lateral veins which can be distinguished well in XPCM images. Therefore, we selected them as materials. A leaf of corn and rice was cut off from whole plant and the cut end was put in tap water for several hours to make sure that it was sufficiently hydrated. Before recording process, its immersed cut end and its apex were cut off again to accelerate the evaporation of water in it. Then, the leaf was fixed on the sample stage.
During the experiment, a High Pressure Sodium Lamp (HPSL) of 150 Watt was fixed about 30 cm away from the sample to accelerate its water evaporating.
Photon energy of 15 keV was chosen. Two CCD, which were 9 cm away from the sample-detector, were used to meet the requirements of high spatial resolution and short exposure time. The position of the sample holder could be adjusted in y and z directions to ensure that the cut end of the sample at bottom could be seen and air had already entered several vessels from the two ends of the leaf. The XPCM images of the sample can also be previewed on PC screen in live mode, in which the air background is not excluded.
3. Results and Discussion
3.1. Preliminary Experimental Results
1) Paraffin sections of the different species were done to know the structure of leaves.
From the pictures of Paraffin sections of rice and corn, there were several gaps in their midvein. The gaps were several times the diameter of the vessels in it, letting the vessels not be recognized. Therefore, midveins of rice and corn are
![]()
Figure 1. Schematic diagram of BL13W1 at SSRF.
not suitable as an observation object. The diameters of vessels in lateral veins of corn leaf were larger than 20 μm, and in XPCM experiment the inflation vessels displayed clearly. Therefore, we looked for bubble extending in lateral veins of corn leaves, also of rice leaves.
Here we show hand cross sections of lateral vein of corn leaves (Figure 2). It can be seen that there were two large metaxylem vessels (V), and one protoxylem lacuna (arrow). In Figure 2(a), the arrow points to a perforated plate of the lacuna. The arrow in Figure 2(b) points to a protoxylem lacuna with white, indicating that it was empty.
2) Experiment of ultrasonic emission
After a rice leaf was cut from whole plant, little ultrasonic emission could be captured within one hour. Considering that leaf damage will increase water evaporation from it, the apex of a leaf was cut. It was true that we detected ultrasonic mission event and after a leaf was cut (Figure 3) from whole plant 10 - 20 min later the signals were the highest. On average, each leaf blade emitted 4 - 7 ultrasonic events. Thus, the result locked the time frame of cavitation event in XPCM experiment. Figure 4 is a waveform of an ultrasonic emission of a rice leaf. It lasted about 50 μs and its frequency is between 60 - 300 kHz.
![]()
Figure 2. Hand cross sections of lateral veins of corn leaves.
![]()
Figure 3. A leaf used in X-ray experiment.
![]()
Figure 4. A waveform of an ultrasonic emission of a rice leaf.
3) Water potential of leaves
The initial water pressures of leaves of rice and corn were lower than −0.5 MPa when they were cut from whole plants.
3.2. Cavitation Event in Intact Conduits by XPCM Experiment: Vessels Were Fully Filled with Air Instantly
Based on knowing the microstructure of the leaves of rice and corn, determining the area of cavitation event in a leaf, and also determining the time frame of cavitation events, we looked for cavitation event in lateral veins of the leaves in XPCM experiment.
In XPCM experiment, the sample stage was fixed in the horizontal direction at the position where a vessel of a lateral vein had been fully filled with air. After that the stage was moved vertically and fixed till water/gas meniscus could be seen. Image sequences were taken with time to record the dehydration process in the field of CCD view. The recording process usually lasted for about tens of minutes to increase the probability of capturing the images of the cavitation occurrence in the selected region.
Series of images of corn leaves were captured with exposure time of 800 ms and time interval of 5 s. After image sequence was finished, we immediately excluded the air background and looked for the images according to what we observed in live mode. Two consecutive images with significant difference were found in the sequence (Figure 5). First, the vessel of diameter d ≈ 40 μm in the right in Figure 5(a), which corresponds to the large metaxylem vessels (V) in Figure 2, had been filled with gas and the ends between adjacent vessel elements can be clearly identified as the white arrows point. The most important is that the narrower adjacent vessel of d ≈ 20 μm in the left (Figure 5(a), arrow w, corresponding to the protoxylem lacuna in Figure 2), is full of water, with the water/air meniscus at the bottom (arrow m). While in the next image (Figure 5(b)), the bubble expanded (arrow g) in more than one vessel element in the whole field of CCD view by over 1300 μm. This means that the bubble at least extended 1170 μm. Thus, the diffusing speed of the gas in the vessel should be faster than 1170 μm/5.8 s ≈ 202 μm/s.
In fact, when we watched the screen of the computer the bubble extended from bottom to top through perforated plates and fully filled the vessel in the whole field of view in a flash (much less than 5 s) in live mode. Comparing Figure 2 and Figure 5, it is true that the cavitation event in Figure 5 happened in such a protoxylem lacuna as the arrow points in Figure 2.
The same phenomenon was captured in rice leaves. Figure 6(a) and Figure 6(b) are two consecutive images recorded in sequence with an exposure time of 1 s and time interval 2 s. The segment of a bubble appeared in a vessel (arrow m), and most of the vessel was full of water (arrow w) (Figure 6(a)). While in the next image (Figure 6(b)), the bubble expanded out of the field of CCD. This also means that the bubble air filled the vessel for an instant.
![]()
Figure 5. A bubble extended for a moment in a corn leaf. Scale = 100 μm.
![]()
Figure 6. A bubble extended for a moment in a rice leaf.
In the XPCM experiment, although it was more easily to obtain the process of bubble filling conduit(s) for a moment, it was harder for us to get the gradual expanding of bubbles.
4. Discussion
Which process of cavitation does the process of vessel fully filling with gas for a moment belong to?
In the light microscopy experiment of sections of xylem of P. orientalis [13] , the first way was characterized by a gradually expanding speed of about 10 μm/s. For the second way, the diameter of the initial torispherical bubble was expanding with a speed of about 0.050 μm/s, then the bubble burst, the increased speed of the bubble edge with blurry edge of air/water was about 50 μm/s, and then the bubble lengthened continuously with a speed of about 10 μm/s, which was the same as the first way. For the third way, the gas diffused with a speed of about 1.0 × 103 μm/s. In the process the edge of air/water was blurry also. The paper suggested that speeds of 50 μm/s and 1.0 × 103 μm/s all corresponded to the explosion event caused by air seeding.
The diffusing speed of 202 μm/s for the gas in the corn leaf was much faster than 50 μm/s and slower than 1.0 × 103 μm/s. In fact, we have pointed out that the process occurred only in a flash, much less than the initially set time interval of 5 s. Thus, we deduce that the gas diffusion should been caused by the bubble explosion at the junction of the two protoxylem lacuna elements (arrow m) in Figure 5(a). Due to the 800 ms exposure time and the relatively rapid gas diffusion rate, it was challenging to capture a blurry boundary of water/gas.
How can this phenomenon be explained?
Based on the analysis by Schenk et al. [23] , when air moves through a pit membrane (or a perforate plate), it should form small bubbles. Suppose an air bubble with atmospheric pressure 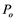 enters a conduit of diameter d = 20 μm which is filled with water at tension, as illustrated in Figure 5. According to the Shen’s paper [11] , as long as the diameter of a gas bubble is less than 7.3 μm, it will break as the absolute xylem pressure drops below
enters a conduit of diameter d = 20 μm which is filled with water at tension, as illustrated in Figure 5. According to the Shen’s paper [11] , as long as the diameter of a gas bubble is less than 7.3 μm, it will break as the absolute xylem pressure drops below 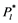 = −0.00973 MPa. From Figure 2(a), there were several patches in the perforate plate area. From Figure 5(b) the appearance of perforate plate area (white arrow) is very dense. Therefore, the diameters of the bubbles that exited from the perforate plate in our XPCM experiment (Figure 5) should be shorter than 7.3 μm. Along with water evaporating from the cut ends of the leaf, which led to a drop in absolute xylem pressure from
= −0.00973 MPa. From Figure 2(a), there were several patches in the perforate plate area. From Figure 5(b) the appearance of perforate plate area (white arrow) is very dense. Therefore, the diameters of the bubbles that exited from the perforate plate in our XPCM experiment (Figure 5) should be shorter than 7.3 μm. Along with water evaporating from the cut ends of the leaf, which led to a drop in absolute xylem pressure from 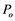 to below −0.00973 MPa, these air bubbles would explode.
to below −0.00973 MPa, these air bubbles would explode.
The situation of the rice leaf in Figure 6 was the same.
In Ponomarenko’s experiment [10] , for their first event (cf Figure 3(b)) the diffusing speed of the bubble was faster than 200 μm/s. For their second phenomenon (cf Figure 3(c)) which resulted in extending of gas patches, the initial extending speed of the bubble was about 140 μm/s. We thought that above two speeds might represent the moving rates of the air of the bubbles after their bursting. For their second phenomenon (cf Figure 3(c)) after the 1.75 s bubble growing speed became less than 30 μm/s. It might represent a gradual moving.
Before an explosion event in a conduit occurs, the pressure 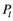 would drop from
would drop from 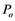 for a period of time. This is why the first acoustic signals were recorded late in our X-ray experiment and others [14] .
for a period of time. This is why the first acoustic signals were recorded late in our X-ray experiment and others [14] .
Except of the cavitation event caused by air seeding, some experiments showed the occurrence of isolated embolized conduit [24] [25] , which might be caused by the growth of pre-existent air nanobubbles in xylem.
Whether an explosion event is the second or the third way of cavitation depends on whether there is a gradual expanding of a small bubble before an explosion event [12] [13] .
Both gradual expanding of bubbles and gas diffusing at high speed in the sections of plants have all been obtained by light microscopy [13] . Then, in synchrotron X-ray experiment of corn and rice leaves gas diffusing at high speed was more easily gotten and it was harder for us to get the gradual expanding of bubbles. Why?
We have known that for the second way of cavitation by air seeding to occur in a conduit of radius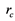 , the radius of a crack
, the radius of a crack 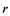 which an air seed moves through should be in
which an air seed moves through should be in 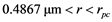 and
and 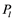 in
in![]() . Here
. Here ![]() is the radius of the pore through which an air seed enters a conduit of radius
is the radius of the pore through which an air seed enters a conduit of radius ![]() in the absolute water pressure
in the absolute water pressure ![]() and it will burst at absolute water pressure
and it will burst at absolute water pressure ![]() with its radius
with its radius![]() . For the first way,
. For the first way, ![]() and
and![]() . For the third,
. For the third, ![]() and
and ![]() [11] [12] . Because the sections of P. orientalis and some corn leaves were taken out of water and dehydrated in air, the pressure
[11] [12] . Because the sections of P. orientalis and some corn leaves were taken out of water and dehydrated in air, the pressure ![]() in the conduits of the samples should drop continuously from
in the conduits of the samples should drop continuously from ![]() and could be at any values lower than
and could be at any values lower than![]() . Hence, as for which way of cavitation will happen only depends on the size of the holes in the walls of the conduits. Because some cracks in the walls of the conduits of the section of P. orientalis were big enough (because of cutting), air could pass them into the conduits directly, or overtime air could enter the conduits through some slightly smaller cracks to form air seeds, leading the first way of cavitation to occur [13] . However, in the X-ray experiment, air was not easily to enter the intact vessels of corn leaves at
. Hence, as for which way of cavitation will happen only depends on the size of the holes in the walls of the conduits. Because some cracks in the walls of the conduits of the section of P. orientalis were big enough (because of cutting), air could pass them into the conduits directly, or overtime air could enter the conduits through some slightly smaller cracks to form air seeds, leading the first way of cavitation to occur [13] . However, in the X-ray experiment, air was not easily to enter the intact vessels of corn leaves at ![]() directly, letting the first way of cavitation to happen harder.
directly, letting the first way of cavitation to happen harder.
For the third way of cavitation to happen, the key factors are that there are intact conduits and the water in them at tension. In the X-ray experiment, most vessel elements of corn leaves which the x-ray was through were intact and the leaves did not wilt, leading the third way of cavitation to be captured more easily. However, only a small number of the sections of P. orientalis had intact conduits, letting the third way not to be captured in most of 29 sections of P. orientalis [13] .
For the second way of cavitation by air seeding, crack radii in the wall of a conduit should be in a narrow range (![]() for
for![]() ). As a result, the second phenomenon rarely occurred in our experiment of the sections of P. orientalis [13] .
). As a result, the second phenomenon rarely occurred in our experiment of the sections of P. orientalis [13] .
From above we suggest that for some whole plant the third way of cavitation by air seeding is priority; the second way by the growth of pre-existent air bubbles, especially by the growth of nanobubbles can occur. After all, which way can happen depends on the structure of pit membrane in xylem wall and on the presence of micro air bubbles in conduits. Studying the difference of structure of pit membrane and the formation of air bubbles in conduit will be helpful for selection of drought-resistant varieties of plants.
5. Conclusion
An explosion event, which can only occur in intact conduit of xylem at water tension, trigs acoustic emission and induces air to diffuse with high speed, simultaneously. By testing ultrasound emission, the time frames of cavitation events of leaves of corn and rice were determined. It is also determined that we would look for bubble extending in lateral veins of corn and rice leaves. Then, synchrotron X-ray phase contrast microscopy was used to capture cavitation event of the leaves. The images show that the phenomenon of gas bubble fully filling conduits for a moment occurs in the leaves. Hence, our theory and experiments interpret the process of cavitation event in xylem, also the functional implications of intervessel connections. Studying the difference of structure of pit membrane and the action of air bubbles in conduit will be helpful for selection of drought-resistant varieties of plants.
Acknowledgements
We thank Professor Zhang Xiangxue for his assistance at making cross sections of lateral vein of corn leaves. This work was financially supported by National Natural Science Foundation of China (#30270343) and Fundamental Research Funds for the Central Universities of China (Grant No. YX 2011-15).
Supplementary Note
There is a mistake in the article (Shen 2020). The formula ![]() should be
should be![]() .
.
NOTES
*Corresponding author.
†Mr. Gao R is old professor and died in 2021.