1. Introduction
The unrestricted usage of chemical agents in plant protection systems causes serious environmental problems, such as agro-pest resistance to substances, dangerous contamination of the environment with residues, and negative effects on crop quality and quantity, as well as human health. Therefore, it is important to use of new plant protection methods against pest insects with various mechanisms of biological action [1] .
Currently, a new approach to insect population management is actively developing, based on the mechanism of manipulations in the communicative behavior of arthropods. It has been established that the transmission of information in insects is provided by chemical substances—exohormones. Such low molecular weight bioregulators include pheromones, substances produced by insects and released into the environment for intraspecific communication. The ecological advantages of pheromones are undoubted, since their action is maximally species-specific, the effective doses of the described substances are minimal, even compared to juvenoids, they are not toxic and do not leave toxic residues in the environment. It should be noted that recently, thanks to the efforts of chemists from various countries, significant progress has been made in the field of identification and chemistry of insect pheromones.
The purpose of this study was to study natural bioregulators—pheromones, attracting substances of the melon fly (Myiopardalis pardalina Bigot.), to optimize the monitoring of phytophages on melon plantations in Uzbekistan.
The melon fly (Myiopardalis pardalina Bigot., 1891) is a two-winged insect from the variegated family, the body of the melon fly is 5 - 6 mm long, pale yellow, and its abdomen is orange in color, the shape of the eggs is oblong (Figure 1). It mainly damages plants of about 125 species, primarily the gourd family (Cucurbitaceae), such as melon (Cucumis melo), watermelon (Citrullus lanatus), cucumber (Cucumis sativus), and pumpkin (Cucurbita pepo).
The melon fly (Myiopardalis pardalina Bigot.) is widespread in some European countries, in particular Azerbaijan, Armenia, Georgia, Cyprus, Turkey, Ukraine and Asia: Afghanistan, India, Israel, Jordan, Iraq, Iran, Lebanon, Pakistan, Saudi Arabia, Syria, Kazakhstan, Kyrgyzstan, Tajikistan, Turkmenistan, and Uzbekistan.
During the year, the melon fly gives 3 - 4 generations. Flies appear at the time of melon flowering. Female flies lay their eggs in the skin of ovaries and in young fruits, as well as on leaves. The larvae penetrate the pulp of the fruit, where they feed juice and seeds, then leave the fruit and go to pupate in the soil.
Spring flight coincides with the period of fruit formation in forage plants. At this time, the temperature of the soil where insects hibernate reaches +20˚C. The flight of the pest is observed from early June to mid-October. They feed on fruit juice. The life span of adults is 2 months. The puncture sites of the pulp can serve as a medium for the development of viral and fungal diseases. The first signs of damage by a melon fly are the appearance of small bumpy spots, or simply bumps in places where the fruits were bitten. Later, after the development of the larvae, internal decay of the fruit begins. Damaged fruits then rot and become unsuitable for further use.
![]()
Figure 1. Imago and pupa of the melon fly (Myiopardalis pardalina Bigot.).
The main control methods for melon fly are based on changing in landing site of the planting material, and such procedures are carried out constantly. Also, the collection of pupae is carried out at the place of planting melons and the destruction of the damaged crop. An effective method is fumigation before dawn and after sunset and covering melons with fabric [2] . In order to limit the further spread of insect pests, there are strict quarantine restrictions. One of the elements of the control program of the melon fly (Myiopardalis pardalina Bigot.) is the mass capture of males, but at the same time, the capture of female flies is no less important for reducing fruit damage [3] [4] .
Thus, integrated pre-harvest methods of insect pest control include the destruction of males, sanitation, protein baits to attract females, and the use of insecticides [5] . The proposed study describes methods for isolating and identifying allelochemicals of the melon fly, developed methods for the synthesis of synthetic analogs of attractants.
2. Area of Study
Identification of the structure of attractive substances from the biomaterial of the melon fly (Myiopardalis pardalina Bigot.). The biomaterial of the melon fly was collected in the experimental plots of melons of the Karakalpak Institute of Agriculture and Agrotechnology (Nukus, Karakalpakstan).
3. Materials and Methods
Chemicals and solvents were purified by standard techniques. For thin-layer chromatography (TLC), silica gel plates Silufol, eluents (ethyl ether-hexane/1:1; hexane-acetone/2:1), compound were visualized by irradiation with iodine vapor. Flash chromatography was performed using silica gel Merck 60 F254, eluents-system of ethyl ether and hexane.
4-methoxybenzaldehyde was purchased (chemical from “Sigma-Aldrich” company) and used without further purification. The melting point temperature was measured using a micro melting point apparatus. The double bond reduction reaction was carried out on a hydrogen reduction apparatus.
GC-MS analysis was performed on an Agilent 8890 GC with split and splitless evaporators used in conjunction with an Agilent 5977B GC/MSD in SIM, SCAN, and electron impact (EI) ionization modes. Analysis conditions: analytical column HP-5ms Ultra Inert 30 m × 250 µm × 0.25 µm; injection volume 1.0 μl; splitless injection mode—evaporator temperature 280˚C; UI liner, splitless, single constriction, fiberglass—gold plated sprayed gasket, Ultra Inert with washer; carrier gas—hydrogen, constant flow, 1.2 ml/min. Thermostat program 60˚C for 1 minute, then 25˚C/min to 170˚C, then 10˚C/min to 310˚C, then hold for 2 minutes. The temperature in the transport line is 280˚C. MS conditions: delay to eliminate solvent effects 3.5 minutes; SCAN data collection mode; gain 1.00; Temperature source 250˚C.
The biomass of insects was maintained under laboratory conditions according to commonly accepted methods.
3.1. Extraction of Allelochemicals Isolated from the Biomaterial of the Melon Fly (Myiopardalis pardalina Bigot.)
The biomaterial of the melon fly was kept in glass containers covered with moistened gauze, with small pieces of melon attached to support the vital activity of insects, kept at room temperature for 72 hours. At the same time, the covering material was kept constantly moistened with a sugar solution. Then, adult melon flies were transferred to a cylindrical structure with removable lids for the exterminating procedure (Figures 2(a)-(d)).
Insect exterminating procedure was carried out under the influence of small doses of diethyl ether. At first, an excited state was observed in the behavior of insects, individuals circled on their backs around themselves, sharply shaking their wings. After that, a temporary stupor of insects was observed. In petrified females of the melon fly the sternal glands were dissected and placed in a bottle with 5 ml of methylene chloride. The extract was kept in a refrigerator for several days (Figure 3(a) and Figure 3(b)).
![]() (a)
(a) ![]() (b)
(b)
Figure 3. (a) and (b) Preparation of extracts of dissected melon fly specimens.
According to the method described above, 5 samples of extracts of the sternal glands of female melon fly were prepared. Then, the extracts were concentrated under water jet pump vacuum for identification analysis.
3.2. Analysis of Allelochemicals Isolated from Melon Fly (Myiopardalis pardalina Bigot.) Biomaterial Using GC-MS Analysis
GC-MS analysis was performed on an Agilent 8890 GC with split and splitless evaporators coupled to an Agilent 5977B GC/MSD in SIM, SCAN, and electron impact (EI) ionization modes.
According to the analysis results, the most significant signals are presented:
3.3. Synthesis of Precursors of a Synthetic Analog of Parapheromone-4-(4-Acetoxyphenyl)-2-Butanone
Synthesis of Z-4-(4-methoxyphenyl)but-3-en-2-one (III)
0.1 M of 4-methoxybenzaldehyde and 0.44 mol of acetone were placed into a beaker with a magnetic stirrer, the contents were diluted with water and stirred at 25˚C - 30˚C. Then, a 10% NaOH solution was gradually added to the reaction mixture, while the reaction mixture was heated to 38˚C. The contents of the glass became cloudy yellow-green. At the end of the reaction, a yellow precipitate formed. To process the reaction product, the reaction mixture was acidified with 10% HCl solution to an acidic reaction with litmus. The precipitate was separated, washed with water. The precipitate formed was dried in air. Yield: 73%. M.p. 58˚C - 60˚C. The progress of the reaction was monitored by TLC, [hexane:acetone/2:1] system, Rf of the reaction product = 0.69.
Synthesis of 4-(4-methoxyphenyl)butan-2-one (IV)
A mixture of catalysts was prepared in a conical flask with a magnetic stirrer attached to a hydrogenation device, for which 0.03 M NiAc2∙4H2O was dissolved in 225 ml of ethanol, then a calculated EDA in ethyl alcohol, at the rate of 0.03 M per substance to be recovered. The system was filled with gaseous hydrogen, after which an alcoholic solution of the recoverable substance 0.03 M III was added. The calculated amount of gaseous hydrogen was passed into the system. After the completion of the reaction, the reaction solution was passed through a column with SiO2 (100/250). The alcohol solution was diluted with water. The organic layer was separated. The aqueous solution was extracted with ether (2 * 50 ml), the organic extracts were washed with saturated NaCl solution. Dried over Na2SO4. Concentrated under water jet pump vacuum. The residue was fractionated by distillation under vacuum oil pump with reflux condenser. B.p. 122˚C - 125˚C/0mmHg Yield: 84%. The progress of the reaction was monitored by TLC, system [ether:hexane/1:1], Rf of the reaction product = 0.55.
Synthesis of 4-(4-methoxyphenyl)-2-methyl-1,3-dioxane (V)
0.01 mol of IV, 0.015 mol of ethylene glycol, 0.001 mol of p-toluenesulfonic acid in 50 ml of benzene were placed in a round-bottom flask with a Dean-Stark nozzle and a reflux condenser and boiled until the calculated amount of water was released. Upon completion, the reaction mixture was diluted with benzene. Washed with soda solution, then with NaCl solution. The organic extracts were dried over Na2SO4. The progress of the reaction was monitored by TLC in the system [ether:hexane/1:1], Rf of the reaction product = 0.62. Yield: 80%.
Synthesis of 4-(4-hydroxyphenyl)butan-2-one (VI)
In a round bottom flask, 0.01 mol V, 0.02 mol HBr, 0.02 mol HAc in 10 ml benzene were mixed and boiled for 6 hours. The reaction mixture was then diluted with water. The organic layer was separated and washed with 20% NaOH solution. The organic layer was separated, acidified with 10% HCl solution. The organic extracts were dried over Na2SO4. The progress of the reaction was monitored by TLC in the system [ether: hexane/2:1], Rf of the reaction product = 0.42. Yield: 53%. A crystalline substance is isolated, with a characteristic fruity odor. M.p. 72˚C - 75˚C.
Synthesis of 4-(4-acetoxyphenyl)-2-butanone (II)
0.01 mol of VI, 0.01 mol of acetic anhydride, 50 ml of ether and 1 - 2 drops of phosphoric acid were placed in a round bottom flask. The mixture was stirred at reflux for 3 hours. Upon completion, the reaction solution was diluted with ether. The organic layer was washed with 5% NaOH solution. Washed with water and dried the organic extract over Na2SO4. The progress of the reaction was monitored by TLC, in the system [ether: hexane/2:1], Rf of the reaction product = 0.33. The residue was fractionated by distillation under vacuum oil pump with reflux condenser. B.p. 125˚C - 128˚C/0mmHg. Yield: 58%.
4. Discussion of Results
It is known that, in fruit flies (Dacini), sex pheromones are usually produced in the rectal glands of males and attract virgin females. The sex pheromones of D. dorsalis and D. cucurbitae act interspecifically, attracting females of other species, which indicates a very similar chemical constitution [6] . The D. cucurbitae male sex pheromone consists of a complex mixture of nitrogen compounds, of which N-3-methylbutylacetamide appears to be the most active in increasing flight and search activity in females [7] .
The sternal glands of female of melon fly were dissected and extracts were prepared from them. Pooled extracts of dissected specimens of melon fly were analyzed by GC-MS. According to the results of the GC-MS analysis, a repetitive signal with a close retention time was initially detected, which is displayed in Figure 4 and Figure 5 of the spectrum.
For fractions with retention time RT(min) = 23.274; RT(min) = 23.683; RT(min) = 23.147; a structure corresponding to RT = 23.147 was revealed, which corresponds to the structure of 1,4-benzenedicarboxylic acid, bis(2-ethylhexyl) ether (I):
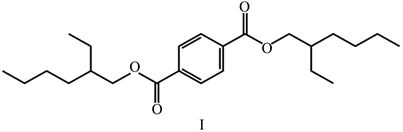
We suppose that the identified compound is a food lure for the Myiopardalis pardalina, and also, this compound has been found in several plants and classified as biologically active compounds, which has influenced on the behavior of living organisms [8] [9] [10] .
![]()
Figure 4. GC-MS analysis of the melon fly extract fraction with retention time RT = 23.274.
![]()
Figure 5. GC-MS analysis of the melon fly extract fraction with retention time RT = 23.147.
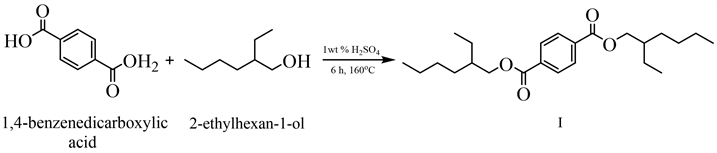
Based on the structural features of the identified component, a method was proposed for obtaining its synthetic analogue of 1,4-benzenedicarboxylic acid, bis(2-ethylhexyl) ether by the esterification reaction of 1,4-benzenedicarboxylic acid and 2-ethylhexan-1-ol.
Also, the authors [11] [12] studied that methyl eugenol and raspberry ketone are parapheromones—allelochemical substances that stimulate intraspecific reactions between males and females in fruit flies.
Based on this, we hypothesize that a pheromone mixture of 4-(4-acetoxyphenyl)-2-butanone (cuelure), methyl eugenol and I, as a synergist, will elicit a behavioral response in Myiopardalis pardalina Bigot.
In this regard, for the implementation of biotesting, we have developed a synthesis route-4-(4-acetoxyphenyl)-2-butanone (II).
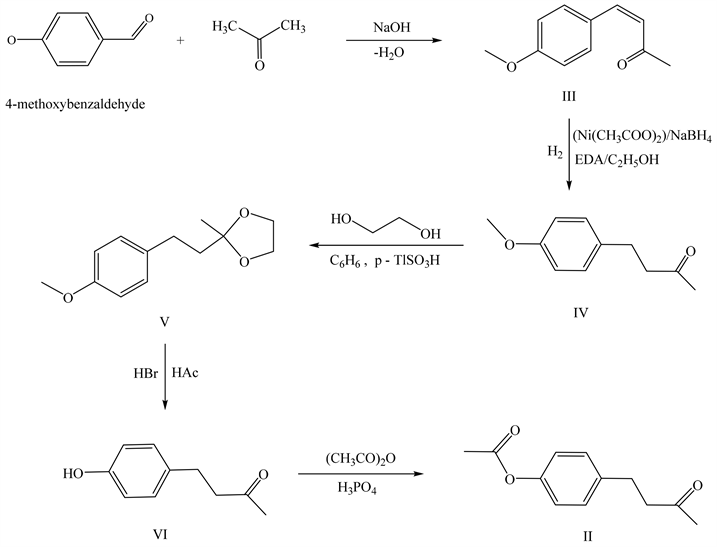
We used 4-methoxybenzaldehyde as the starting compound. From which Z-4-(4-methoxyphenyl)but-3-en-2-one (III) was successively obtained by coupling with acetone in 73% yield; which, by hydrogenation, was converted to 4-(4-methoxyphenyl)butan-2-one (IV) in 80% yield; to protect the existing keto-group, diethylene glycol was used; 4-(4-methoxyphenyl)-2-methyl-1,3-dioxane (V) was converted by saponification into 4-(4-hydroxyphenyl)butan-2-one (VI) in 53% yield; which, by acetylation reaction, gave the desired 4-(4-acetoxyphenyl)-2-butanone (II) in 58% yield.
5. Conclusions
To maintain the optimal phytosanitary condition of agricultural crops, there is a system of agrotechnical and chemical practices that prevent crop losses, without the negative impact of the means used on humans and the environment. One of the main stages of which is the timely detection of pests and their maintenance at a safe level, that is, quantitative and qualitative monitoring. Monitoring is carried out through the use of substances that bioregulate the behavioral functions of insects, which are based on allelochemicals.
In this study, natural bioregulators were studied—pheromone simulators, attracting substances of the melon fly, for subsequent optimization of phytophage monitoring on melon plantations in Uzbekistan.
As a result of the studies of natural bioregulators of the melon fly (Myiopardalis pardalina Bigot.), the structure of the food attractant of the melon fly, 1,4-benzenedicarboxylic acid, bis(2-ethylhexyl) ether (I), was revealed, and taking into account the structural features of the identified component, was a method for obtaining its synthetic analogue is proposed. Based on the literature data, a mixture of pheromone imitators was proposed to attract melon flies to pheromone traps, consisting of a mixture of parapheromones: (4-(p-acetoxyphenyl)-2-butanone (II), methyleugenol and food attractant-1,4-benzenedicarboxylic acid, bis(2-ethylhexyl) ether (I), which creates a synergistic effect in the mixture and leads to a behavioral response. Also, alternative routes for the synthesis of synthetic analogs of attractants have been developed.
Biotesting of isolated substances and their synthetic analogues, to identify an active behavioral response, is ongoing.