Cutaneous Symptoms Revealing M5 Acute Myeloid Leukemia: A Case Report ()
1. Introduction
Acute leukemia (AL) constitutes a heterogeneous group of malignant hemopathies characterized by the clonal and uncontrolled proliferation of hematopoietic precursors blocked in their differentiation [1]. They represent between 10 and 15% of hematological malignancies with an incidence rate standardized to the world population of less than 6/100,000 inhabitants/year [2]. Currently, clinical pediatric AML studies are conducted separately according to the AML subtypes: de novo AML, acute promyelocytic leukemia (APL), and myeloid leukemia with Down syndrome (ML-DS).
Cutaneous localizations are not uncommon in acute myeloid leukemia (AML) but are exceptionally revealing. They are most often avoided as a poor prognosis. We report a case of an 11-month-old male who presented with skin nodules revealing M5 acute myeloid leukemia.
2. Observation
This is an 11-month-old male infant from a non-consanguineous marriage with a history of neonatal care for maternal-fetal infection and familial atopy. Admitted for management of a right supranodular skin nodule associated with papulonodular lesions, all evolving in a context of fever and asthenia (Figure 1, Figure 2).
The clinical examination found a conscious infant, hemodynamically and respiratory stable, feverish at 38.5˚C, asthenic, pale with discolored conjunctiva.
![]()
Figure 1. Diffuse purplish papulo-nodular lesions on the face.
![]()
Figure 2. Diffuse purplish papulo-nodular lesions on the face, gum, trunk, back and seat.
Diffuse purplish papulo-nodular lesions and ecchymosis on the face, gum, trunk, back and seat, evolving for two weeks, related to leukemides (Figure 1, Figure 2). Abdominal examination reveals moderate splenomegaly and ENT examination reveals gingival hypertrophy and hard right supraorbital swelling. The biological assessment revealed on the Blood Formula Count: Pancytopenia with normochromic normocytic anemia Hemoglobin at 8.7 g/dl, VGM at 72.5, TCMH at 25.5, Aregenerative with a reticulocyte count of 12,300 G/L and absence of blasts on the blood smear.
The bone marrow objectified a blast rate at 03%, Granular neutrophils at 60%, Erythroblasts at 23%, Lymphocytes at 06%, Plasmocytes at 03%, Monocytes at 01% and the onco-hematologic karyotype was without abnormality.
Considering the right supraorbital swelling, a brain scan was completed for a neuroblastoma mass, which showed bone thickening of the right orbit with rupture of the cortical bone in places and periosteal reaction. The dosage of urinary catecholamines came back negative and the tumor lysis assessment was unremarkable. Abdominal ultrasound revealed a slight homogeneous hepatosplenomegaly. Skin biopsy supported skin infiltration with leukemia/hematoderma with an immunohistochemically study showing that tumor cells express LCA (CD45), CD123, CD 56, CD68, CD15 and CD4. In addition, CD1a, CD3, PAX5, CD30, chromogranin, synaptophysics, desmin and myogenin are negative (Figure 3).
During his hospitalization, the patient presented a macrophagic activation syndrome with fever at 39˚C, splenomegaly with biology pancytopenia, a collapsed fibrinogen level at 1 g/l, triglycerides at 6.16 g/l, ferritinemia elevated to 5855 ug/L and an elevated LDH level to 3098 IU/L. The patient received boluses
![]()
Figure 3. Anatomo-Pathological sections showing CD56, LCA, CD4 and CD15 all positive.
of intravenous corticosteroid therapy for 3 days at a dose of 30 mg/kg/day, then relayed by oral corticosteroid therapy at a dose of 2 mg/kg/day for 7 days with progressive reduction. The clinical biological evolution was favorable.
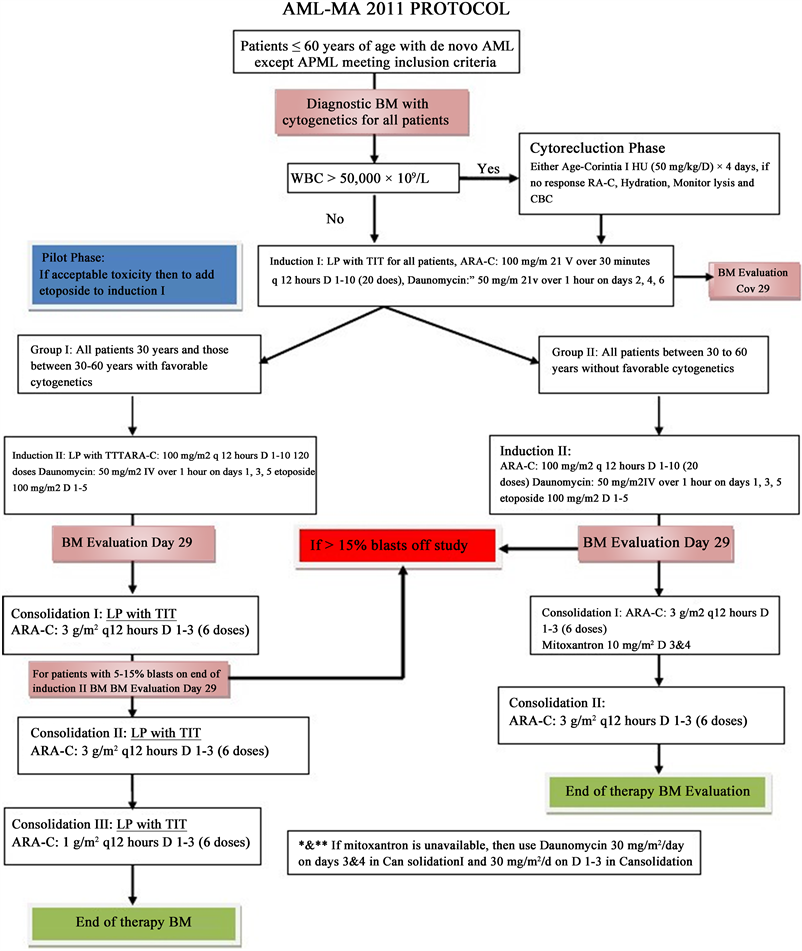
Taking into account the clinical symptomatology of our patient, as well as the results of the immunohistochemistry, the diagnosis of a blast plasmacytoid dendritic cell tumor was retained and the infant was treated according to the AML-MA 2011 protocol (Annex 1), based on Daunorubicin,
Aracytine and L-Asparginase. With initially a good clinical evolution (disappearance of leukemides) then clinical-biological recurrence after the end of the last treatment: Consolidation III (Annex 1) Following the relapse of the skin lesions, the patient was put on intensive chemotherapy based on Aracytine and Etoposide with preparation for a bone marrow transplant. Unfortunately, the death occurred as a result of post-chemotherapy complications.
3. Discussion
Acute leukaemia is 30% - 40% of all malignancies in childhood. It is an aggressive leukaemia cell infiltration into the epidermal, dermis, and subcutis layers. The frequency ranges from 3% to 30% depending on the type of leukaemia and seems to be higher among children than adults are, as many are as 25% - 30% of infants with congenital leukaemia develop skin involvement [3] [4].
In the French ELAM02 cohort, skin involvement was significatively more prevalent in infants occurring in 14.5% of children less than 2 years old compared to 2.6% of older children [5] [6].
The incidence of leukemic cell infiltration on the skin is infrequent, can occur in all types of leukaemia. Leukaemia cutis mostly found in AML M4 and M5 about 10% - 50%, while in AML M0, M1, M2, M3 to 10%. No data on leukaemia cutis related to a specific race, sex, and age. Classification of leukaemia cutis can be both specific and non-specific (leukemoid) lesions.
The diagnosis of acute leukaemia is based on clinical symptoms, complete blood tests and peripheral blood smear examination. The diagnosis must be established based on the study of bone marrow aspiration. The features of skin lesions vary, generally appearing as macules, papules and multiple nodules, 1 - 2.5 cm in diameter, firmly defined, palpable as solid, supple, no itchy and painless.
Sometimes plaques, ulcers, bullae and somewhat rare as palpable purpura and may occur as single or generalized lesions.
In AML M5, lesions tend to be large and purplish, the skin thickens mainly on the face. While in AML M4 are nodules (70%), papules (52%), ecchymosis (26%), placards (22%), purpura (17%), and macules (13%).
Leukaemia cutis has no specific predilection; the same distribution over the whole body can be a single lesion or spread to 70% of the body surface.
In 50% of cases, AML M4 and M5 are common gingival hypertrophy as a result of leukaemia infiltration [6] [7] [8].
The time between the diagnosis of leukaemia and the development of leukaemia cutis varies. In the study of Kang et al., the vast majority (95%) of LC lesions developed after the diagnosis of leukaemia or showed concurrent involvement [9].
This presentation is associated with other extra-medullary organ involvement in AML and is negatively associated with prognosis [6] [10]. However, occasionally leukaemia infiltrates occur concomitantly with systemic leukaemia, as in our patient, and may even precede peripheral blood or bone marrow manifestations.
In such cases, the diagnosis of leukaemia cutis is difficult, and immunohistochemical analysis of skin lesion specimens is especially useful. Horlick et al. reported a case of acute myelomonocytic leukaemia that presented as a clinically benign-appearing cutaneous eruption four months before evidence of leukaemia could be detected in the peripheral blood [11]. Leukaemia cutis also occurs in patients with myelodysplastic syndrome (MDS). Fifty biopsy-proved cases of leukaemia cutis reported by Longacre and a1 [12] included 13 patients with MDS.
Leukaemia cutis is a local manifestation of an underlying systemic process; therefore, treatment should be directed at eradicating the leukemic clone by using systemic chemotherapy. Treatment should be tailored according to AML subtype and by the patient’s ability to tolerate the treatment. Management of leukaemia includes curative and supportive therapy. Curative therapy aims to cure leukaemia, a form of chemotherapy that includes the induction phase, consolidation, intensification, central nervous system prophylaxis, and maintenance. The treatment protocol used is the AML-MA 2011 protocol for non-lymphoblastic leukaemia with methotrexate intrathecal, Daunomycin, Aracytine, Dexamethasone, and L-Asparginase (Annex 1).
Supportive therapy includes the treatment of other diseases that accompany leukaemia and treatment of complications such as blood transfusion, antibiotic or antifungal therapy as indicated, good nutrition and psychosocial aspects approach [13] [14]. Our case is acquired with anaemia, thrombocytopenia, so a packed red cell (PRC) and thrombocyte concentrate (TC) transfusion are done for the preparation of chemotherapy.
4. Conclusions
The cutaneous manifestations associated with hematological malignancies are extremely diverse. Their recognition has a double interest. On the one hand, they sometimes allow the diagnosis of an unrecognized hemopathy and on the other hand, certain cutaneous lesions can mean a prognostic turning point of the malignant hemopathy.
Leukaemia cutis is uncommon as the presenting feature of AML and is associated with an unfavourable prognosis. There is no specific treatment for cutaneous leukaemia other than palliation and symptomatic relief. The 2-year survival rate for the unfavourable prognostic group is 10% - 20%.
Annex 1. AML-MA 2011 Protocol