Synthesis of Some New Bispyrazolidine Derivatives for Biological Interest ()
1. Introduction
Tricyclic pyrazole derivatives were described as inhibitor of growth of Bacillus subtilis, Pseudomonas fluorescens, Staphylococcus aureus and KB cells at moderate concentrations [1]. Many of 4-alkyl and 4,4’-dialkyl-1,2-bis(4-chlorophenyl) pyrazolidine-3,5-dione derivatives demonstrated good activity against MurbB in vitro and low MIC values against Gram-positive bacteria, particularly penicillin-resistant streptococcus pneumoniae (PRSP) [2]. Moreover, 4,4’-(arylmethylen) bis(1H- pyrazol-5-ols), were evaluated for in vitro antiviral activity against peste des petits ruminants virus (PPRV). One of these series emerged at the most interesting compound exhibiting excellent antiviral activity against PPRV and was found to be more potent than the standard drug [3]. Asma Samaunnisa [4] also reported that a set of novel of 2,6-dimethyl-1,4-dihydopyridine-3,5-yl-bis[carbonyl-2-(phenyl)] pyrazolidine-3,5-diones], were subjected to in vivo anti-inflammatory and analgesic activities. Activity of all the derivatives was compared with the chosen standards namely indomethacin and tramadol for anti-inflammatory and analgesic activity respectively using dimethylsulfoxide as control. Almost all the derivatives exhibited significant activity. Moreover, the above compounds [5] were also subjected to anticonvulsant and sedative activities screening. Methods: Anticonvulsant activity was tested using two models namely Maximal electroshock (MES) induced convulsion method and Pentylenetetrazole (PTZ) induced convulsion method. Sedative activity was also screened with two models viz. locomotor activity using actophotometer in mice and anxiolytic activity using the elevated plus maze in rats. Phenytoin, diazepam and chlorpromazine, diazepam were used as standards for anticonvulsant and sedative activities respectively. Results: At both the doses of 200 mg/kg and 400 mg/kg, all the tested derivatives exhibited significant anticonvulsant activities. Conversely, none of the derivatives exhibited better sedative activity which can be compared with the standard. In view of these observations, the intention was directed to synthesize some new bispyrazolidine derivatives of expected biological interest.
2. Experimental
All melting points are uncorrected. IR spectra were recorded (KBr) with a Perkin-Elmer 1430 spectrophotometer. 1H NMR spectra were obtained on Varian EM 399.65 MHz equipment. MS spectra were recorded with a Jeol the MS route JMS.600 H.
2.1. Preparation of Substituted 2,3-Diaza-1,3-butadienes (1) and/or (2)
Aromatic azines were prepared according to the known procedures by condensation of aromatic aldehydes or acetophenone and hydrazine sulphate.
2.2. Preparation of Bisnaphth[2,3-c]pyrazolidinetetrone (3a-f) and (4)
General procedure:
A mixture of aldazine or acetophenonazine (1 and/or 2, 1 mmole) and 2,3-dichloronaphthoquinone (2 mmole) was heated to 100˚C for few hours and then refluxed in 10 ml dry benzene for 10 - 13 hrs. The solution was concentrated near dryness and diluted with methanol and the separated products were filtered off and crystallized from ethanol or chloroform. Yield 45% - 65%.
2.2.1. Adduct (3a), R = -C6H5: 5’,7’,13’,15’-Tetrachloro-6,14-diphenyl- bisnaphth[2,3-c;2,3-g]perhydropyrazolo[1,2-a]pyrazole- 5,8,13,16-tetrone
Red substance, m.p. 197˚C, obtained from benzalazine and 2,3-dichloronaphth- oquinone in 52% yield. Anal Calcd. For C34H20Cl4N2O4 (662.32): C, 61.65; H, 3.04; Cl, 21.41; N, 4.23. Found: C, 61.90; H, 3.24; Cl, 21.65; N, 4.36. IR (cm−1): ν = 1670 (>C=O); 1270 (C-N); 740 (C-Cl). 1H NMR (CDCl3): δ = 4.7 (s, 2H, 2CH-N), 7.24 - 8.3 (m, 18H, Ar-H). MS m/z (%): [M+-4HCl-2C6H5-2H] 359 (0.2).
2.2.2. Adduct (3b), R = -C6H4-p-OCH3: 5’,7’,13’,15’-Tetrachloro-6,14-di- (p-methoxyphenyl)bisnaphth[2,3-c;2,3-g]perhydropyrazolo-[1,2-a] pyrazole-5,8,13,16-tetrone
Violet brown substance, m.p. 160˚C, obtained from anisalazine and 2,3-dichlor- onaphthoquinone in 65%. Anal Calcd. For C36H24Cl4N2O6 (722.36): C, 59.85; H, 3.35; Cl, 19.63; N, 3.91. Found: C, 60.15; H, 3.50; Cl, 19.75; N, 3.94. IR (cm−1): ν = 1680 (>C=O); 1440 (C-H bending of CH3), 2880 (w-CH stretching of CH3); 1270 (C-N); 700 (C-Cl). 1H NMR (CDCl3): δ = 3.9 (s, 6H, 2-OCH3), 4.5 (s, 2H, 2CH-N), 7 - 8.2 (m, 16H, Ar-H). MS m/z (%): [M+-2C6H4-OCH3-C10H4O2Cl2-5H] 276 (11.2).
2.2.3. Adduct (3c), R = -C6H4-o-OH: 5’,7’,13’,15’-Tetrachloro-6,14- di(o-hydroxyphenyl)bisnaphth[2,3-c;2,3-g]perhydropyrazolo[1,2-a]pyrazole-5,8,13,16-tetrone
Brown red substance, m.p. 170˚C, obtained from o-hydroxy-benzalazine and 2,3-dichloronaphthoquinone in 45% yield. Anal Calcd. For C34H20Cl4N2O6 (694.30): C, 58.81; H, 2.90; Cl, 20.42; N, 4.03. Found: C, 58.99; H, 3.0; Cl, 20.29; N, 4.20. IR (cm−1): ν = 1670 (>C=O); 1270 (C-N); 720 (C-Cl); 3480 (-OH). 1H NMR (DMSO): δ = 4.5 (s, 2H, 2CH-N), 7.0 - 8.2 (m, 16H, Ar-H), 9.0 (s, 2H, 2OH). MS m/z (%): [M+-H2O-C6H5-HCl] 562 (2.4).
2.2.4. Adduct (3d), R= -C6H4-p-Cl: 5’,7’,13’,15’-Tetrachloro-6,14- di(p-chlorophenyl)bisnaphth[2,3-c;2,3-g]perhydropyrazolo[1,2-a] pyrazole-5,8,13,16-tetrone
Brown red substance, m.p. 220˚C, obtained from p-chloro-benzalazine and 2,3-dichloronaphthoquinone in 49% yield. Anal Calcd. For C34H18Cl6N2O4 (731.20): C, 55.84; H, 2.48; Cl, 29.08; N, 3.83. Found: C, 56.05; H, 2.60; Cl, 28.99; N, 3.97. IR (cm−1): ν = 1680 (>C=O); 1270 (C-N); 730 (C-Cl). MS m/z (%): [M+-4O-H+] 666.1 (0.4).
2.2.5. Adduct (3e), R= -CH=CH-C6H5: 5’,7’,13’,15’-Tetrachloro-6,14-di- (2-phenylethenyl)bisnaphth[2,3-c;2,3-g]perhydropyrazolo[1,2-a] pyrazole-5,8,13,16-tetrone
Light green substance, m.p. 185˚C, obtained from cinnamaldazine and 2,3-dichloronaphthoquinone in 45% yield. Anal Calcd. For C38H24Cl4N2O4 (714.40): C, 63.88; H, 3.39; Cl, 19.84; N, 3.92. Found: C, 64.14; H, 3.54; Cl, 20.0; N, 4.08. IR (cm−1): ν = 1670 (>C=O); 1440, 950 (CH bend of substituted alkene); 1270 (C-N); 700 (C-Cl). MS m/z (%): [M+-2CH=CH-C6H5-C10H4O2Cl2-2CO+] 225.8 (80.9).
2.2.6. Adduct (3f), R= -C4H3O: 5’,7’,13’,15’-Tetrachloro-6,14- di(2-furyl)-bisnaphth[2,3-c;2,3-g]perhydropyrazolo[1,2-a] pyrazole-5,8,13,16-tetrone
Yellowish green substance, m.p. 200˚C, obtained from furfuralazine and 2,3-dichloronaphthoquinone in 40% yield. Anal Calcd. For C30H16Cl4N2O6 (642.22): C, 56.10; H, 2.51; Cl, 22.08; N, 4.36. Found: C, 56.32; H, 2.68; Cl, 22.28; N, 4.50. IR (cm−1): ν = 1675 (>C=O); 1270 (C-N); 1180 - 1130 (-C-O-C cyclic); 700 (C-Cl). 1H NMR (CDCl3): δ = 4.7 (s, 2H, 2CH-N), 7.2 - 8.18 (m, 14H, Ar-H). MS m/z (%): [M+-2C4H3O-C10H4O-Cl] 316.5 (5.8).
2.2.7. Adduct (4): 5’,7’,13’,15’-Tetrachloro-6,14-di(methyl, phenyl)-bis-naphth[2,3-c;2,3-g]pyrazolol[1,2-a]pyrazole- 5,8,13,16-tetrone
Green substance, m.p. 190˚C, obtained from azine of acetophenone and 2,3-dichloronaphthoquinone in 40% yield. Anal Calcd. For C36H24Cl4N2O4 (690.38): C, 62.63; H, 3.51; Cl, 20.53; N, 4.06. Found: C, 62.85; H, 3.65; Cl, 20.40; N, 3.96. IR (cm−1): ν = 1670 (>C=O); 1270 (C-N); 1400 (CH bending of CH3); 720 (C-Cl). 1H NMR (TFA): δ = 1.3 (s, 6H, 2CH3), 8.0 - 8.3 (m, 18H, Ar-H). MS m/z (%): [M+-C6H5-2CH3+3H+] 586.5 (2.6).
2.3. Antimicrobial Activity
The fungal species were grown in sterilized 9.0 cm Petri dishes containing potato’s dextrose agar (PDA) supplemented with 0.05% chloramphenicol to suppress bacterial contamination (Al-Doory, 1980). From these culture, agar discs (10 mm diameter) containing spores and hyphae were transferred aseptically to screw topped vials containing 20 ml sterile distilled water. After thorough shaking 1 ml samples of the spores suspension were pipetted into sterile Petri dishes, followed by the addition of 15 ml liquefied PDA medium was then left to solidify. The tested compounds were dissolved in ethylene glycol to give 2.0% concentration. Antifungal activity was determined according to the method by Bauer et al. (1966) [6] using 3 mm diameter filter paper discs (Watmann No. 3) loaded with 10 µL of the solution under investigation (2.0%). The discs were placed on the surface of the fungal cultures which were incubated at 30˚C. The diameter of the inhibition zone around each disc was measured (c.f. Table 1). The previous method was used for determining antibacterial activity [7] (c.f. Table 2).
3. Results and Discussion
A cyclic diene system containing N or O does not appear to undergo normal Diels Alder reactions [8]. Wagner [9] found that heating one mole of benzalazine with 2 moles of maleic anhydride to 100˚C in dry benzene for several hours gave an addition compound through simultaneous 1,3 and 2,4 addition of 2 moles of
![]()
Table 1. Fungicidal activity is expressed as inhibition zone in mm of compounds (3a-f) and (4).
![]()
Table 2. Bactericidal activity is expressed as inhibition zone in mm.
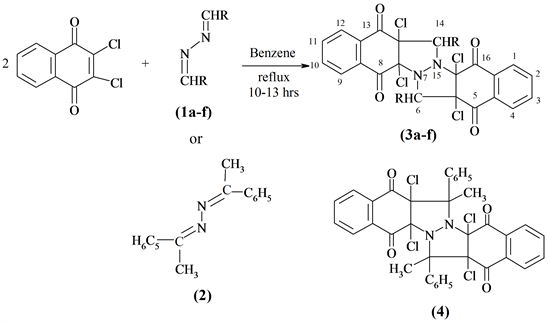
Scheme 1. R = -C6H5 (1a, 3a); -C6H4-p-OCH3 (1b, 3b); -C6H4-o-OH (1c, 3c); -C6H4-p-Cl (1d, 3d); -CH = CH-C6H5 (1e, 3e); -C4H3O (1f, 3f).
maleic anhydride to benzalazine which was designated as criss-cross addition. Moreover, aromatic 1,4-disubstituted-1,4-diazbuta-1,3-dienes with thiocyanates in glacial acetic acid via criss-cross cycloaddition produced the corresponding perhydroimidazo[4,5]imidazole-2,5-dithione [10]. Similarly and continuation to my previous work [11] [12] [13] [14] [15], aldazines (1a-f) such as benzalazine, anisalazine, o-hydroxybenzalazine, p-chloro-benzalazine, cinnamaldazine, furfuralazine and/or acetophenonazine (2) were allowed to react with 2 moles of 2,3-dichloronaphtho-1,4-quinone in dry benzene and afforded bispyrazolidines (3a-f) and (4) (Scheme 1).
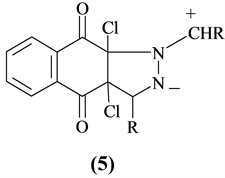
Huisgen [16] suggested that criss-cross reaction can best be represented by series of two [3+2]-cycloaddition steps. Azomethine imine (5) was postulated as a key intermediate [8]. The compounds produced satisfactory results for elemental and spectral analysis.
As for their fungicidal activity, all compounds (3d) and (4) were more active against Asp. fummigatus than others, whereas compounds (3b, c, d) and (4) had a greater activity against Asp. flavous.
And for their bactericidal activity, while most compounds exhibited a remarkable bactericidal activity against B. cereus and M. roses, compounds (3b, c) and (4) had a strong activity against M. luteus and compounds (3a, c, e, f) were active against S. aureus.
4. Conclusion
The title compounds are synthesized successfully through simple novel and one pot reaction from substituted 2,3-diaza-1,3-butadienes and 2,3-dichloro-1,4- naphthoquinone. These compounds also exhibited an interesting fungicidal activity and strongly to remarkable bactericidal activity against some tested micro-organisms.