The kinetics of the periodate oxidation of chromium(III)-complex, [Cr
III(NTA)(Ala)(H
2O)]-(NTA = Nitrilotriacetate and Ala =
ß-alanine) to Cr(VI) have been carried out for the temperature range 15
°C - 35
°C under pseudo-first order conditions,
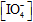
>> [complex]. Reaction obeyed first order dependence with respect to
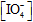
and [Cr(III)], and the rate of reaction increases with increasing of pH for the range 3.40 - 4.45. Experimentally, the mechanism of this reaction is found to be consistent with the rate law in which the hydroxo species, [Cr
III(NTA)(Ala)(OH)]
2- is considerably much more reactive than their conjugate acid. Δ
H* and Δ
S* have been calculated. It is proposed that electron transfer occurs through an inner-sphere mechanism
via coordination of

to chromium(III).