Food and Nutrition Sciences
Vol.5 No.2(2014), Article ID:42103,6 pages DOI:10.4236/fns.2014.52019
Nootropic Activity of Caralluma fimbriata Extract in Mice
1Green Chem, Bangalore, India; 2Department of Pharmacology, AISSMS College of Pharmacy, Pune, India; 3Institute of Food, Brain & Behaviour, Oxford, UK.
Email: *paulrclayton@gmail.com
Received September 30th, 2013; revised October 30th, 2013; accepted November 7th, 2013
ABSTRACT
We investigated the effects of a standardized extract of Caralluma fimbriata Wall (CFE) on learning and memory in mice using various behavioural models. Unusually, CFE exerts both nootropic and anxiolytic effects.
Keywords:Caralluma; Ayurvedic; Nootrope; Anxiolytic; Obesity; Alzheimer’s
1. Introduction
The prevalence of cognitive impairment increases exponentially with advancing age [1,2], and the numbers of affected individuals are increasing due to demographic and other trends. Anxiety frequently presents as a comorbidity [3], and as the anxiolytic drugs currently available generally have sedative properties, this poses problems of clinical management.
We initiated a search for herbs with anxiolytic, nonsedative properties in the Ayurvedic literature, which cites a number of plants as being useful in the treatment of mental symptoms [4]. Some of these have already been examined and found to have significant pharmacological activity [5-8]; specific phytochemicals have been identified as the active constituents [9-11].
Caralluma fimbriata Wall (CF) (Asclepiadaceae), an edible succulent, grows wild all over India and is a traditional food plant. Its phytochemical ingredients include pregnane glycosides, flavone glycosides, megastigmane glycosides, saponins and various flavonoids [12]. Although many of these compounds exert anxiolytic effects [13-15], CF is not known to induce sedation. We investigated its activity on cognition and anxiety in a mouse model.
2. Methods
2.1. Validation of Active
2.1.1. Plant Material and Preparation of Extract
A voucher specimen of Caralluma fimbriata was collected January 14th 2008 at 200 metres altitude in the Kolli Hills Forest in Tamil Nadu, India. It was deposited and identified at the Herbarium of the National Institute of Science Communication and Information Resources (NISCAIR) New Delhi, and registered as NISCAIR/ RIIMD/Consult/06-7/945/129/02 on 25th January 2008. Aerial parts of Caralluma fimbriata Batch CFH/7030 were dried, powdered using sieve 355 and extracted using a hydro-alcoholic mixture (70:30 ethanol:water). This was dried to produce Caralluma fimbriata extract (CFE) containing 25% of pregnane glycosides minimum. These were assayed using HPLC (instrument Shimadzu LC-10 A VP Gradient system) and HPLC Column: Phenomenex C-18, Luna, -SS column 250 mm × 4.6 mm, 5-µ particle size. The mobile phase used was (A) water and (B) acetonitrile at a flow rate of 1.0 ml/min, screened at 205 nm.
2.1.2. HPTLC Fingerprinting of C. fimbriata Samples
Detection by vanillin sulphuric acid reagent.

A. Fresh Herb; B. Dried Herb; C. Extract CFE/7025; D. Extract CFE/WS/06/65% (Working Standard equated to Reference Standard); E. Slimalumaside—A Reference Standard (SLMA/REF/03).
2.1.3. HPLC Analysis
Caralluma fimbriata extract, batch number CFE/10050. SLIMALUMASIDE A, batch number SLMA/REF/04.

2.2. Pharmaceutical Reference Materials
2.2.1. Chemicals and Drugs
Piracetam (PT) syrup (Nootropil, UCB, Batch noV007003) and diazepam injection (Calmpose, Batch no 9059221) were used as reference standards; Piracetam as a recognised nootrope and diazepam as an anxiolytic. All other chemicals were AR grade.
2.2.2. Preparation of Drug Solutions
CFE was dissolved in distilled water to prepare a stock solution from which working solutions allowing dosing at 100, 250, 500 and 1000 mg/kg were prepared. Doses were administered by gavage.
2.3. Animals
Male Swiss albino mice (18 - 22 g) used for the study were purchased from approved supplier (Yash farms, Pune) and maintained at 25˚C ± 2˚C and relative humidity of 45% to 55% under standard environmental conditions (12 h light 12 h/dark cycle). The animals had free access to standard food (Chakan Oil Mills, Pune) and water ad libitum. Institutional Animal Ethics Committee (IAEC) approved the protocol (CPCSEA/IAEC PC-01/ 032K7). All experiments were carried out between 12:00 - 16:00 h.
2.4. Acute Toxicity
Male albino mice (18 - 22 g) were subjected to acute toxicity studies as per OECD 2001 guidelines (AOT 425). The mice were administered with the different doses of CFE or distilled water (10 ml/kg). Dose progression/ reduction was carried out following AOT-425 guidelines.
2.5. Acute Effects of CFE
2.5.1. Object Recognition Test
This was carried out in a multiple open-field box provided by V.J. Instruments, India. Before training, mice were individually habituated by allowing them to explore the open-field box for 2 min on the day prior to testing. During training sessions, the first trial (T1) was conducted 60 min after drug administration. Two identical objects were placed in opposite corners of the box and the time taken by each mouse to complete 20 s of object exploration was recorded. Exploration of the object was considered to be when the head of the animal was facing less than 2 cm from the object or touching the object. Animals were returned to their home cages immediately after training. The second trial (T2) was performed 90 min after T1. A new object replaced one of the objects used in T1 and mice were left in the box for 5 min. Times spent exploring the familiar (F) and new objects (N) were recorded separately and discrimination index (D) was calculated as (N − F)/(N + F). The object was changed randomly and the apparatus thoroughly cleaned between trials to avoid place reference and the influence of olfactory stimuli [16,17].
2.5.2. Locomotor Activity
Locomotor (horizontal) activity was measured using a digital actophotometer (Space-lab, India). Mice were divided into six groups and subjected to respective drug treatment. 60 min after drug administration, mice were placed individually in the actophotometer for 5 min and activity scores obtained. Diazepam (2 mg/kg, i.p.) was used as reference standard [18].
2.6. Chronic Effects of CFE
2.6.1. Morris Water Maze
A 100 cm diameter circular tank was used, made of lightimpervious material and filled to 30 cm with water (25˚C ± 2˚C) rendered opaque with a food-grade lipid/water emulsion. The tank was divided into four equal quadrants with a small platform concealed 2 cm below the water level, placed initially at a fixed position and subsequently randomly to assess the effect of the drugs on spatial reference and spatial working memory [19,20]. Using the standard pre-selection test, mice were released into the tank to find the platform. Mice which failed to locate the platform in the allotted time of 90 s or refused to search were excluded [20]. Mice were then given CFE or vehicle for 21 days.
Evaluation for Learning Facilitation Activity
Selected mice were released into the tank individually and allowed to find the hidden platform at either fixed or variable locations within a time limit of 90 s. Distances travelled and times taken to find the hidden platform (escape latency) at a fixed location (working memory) or variable location (short term spatial memory) were recorded daily for 21 days, 60 min after drug administration [20].
2.6.2. Elevated Plus Maze (EPM)
An elevated plus maze (V.J. Instruments, India) consisting of two open arms (50 × 10 × 40 cm) and two enclosed arms (50 × 10 × 40 cm) was used, elevated to the height of 40 cm. Mice were placed individually in the centre of the EPM facing an enclosed arm. Time spent by the mouse during the next 5 min on the open and enclosed arms was recorded. The mice received drug treatment for 14 days. Exploratory activity was measured on 2nd, 7th and 14th day at 60 min after drug administration; time spent in the open arm is indication of anxiolytic activity [16,17].
2.6.3. Radial Arm Maze
A radial arm maze (Vijay Instruments, Pune, India) with an octagonal central hub of 30 cm in diameter and radial arms of 55 × 10 × 40 cm was used. Food containers were mounted at 30 cm into each arm [20].
2.7. Pre-Experimental Food Intake
Mice were provided with excess of weighed food; food remaining at 24 hours was weighed to ascertain daily food intake. This was repeated for 7 days, and average daily food intake assessed. During the experiment 85% of this quantity was provided to generate food-motivated performance.
2.8. Experimental Procedure
This comprised two distinct trials, wherein a food pellet was placed in a fixed arm for spatial reference memory evaluation and in the variable arm for evaluation of spatial working memory. Each mouse placed on the central hub was allowed to choose any arm to obtain food. The session was completed once the mouse had searched all cups or after 3 minutes, when the mouse was returned to its home cage. Successful retrieval of food was recorded as a correct entry; repeat visits to previously emptied cups as errors. Mice were designated trained once they found all food with maximum one re-entry on three consecutive days. Latency to find food, number of correct entries before first re-entry and the number of re-entries of trained mice were measured to evaluate performance. There was a one-hour interval between spatial reference and working memory evaluation. The apparatus was thoroughly cleaned between trials to avoid place preference and the influence of olfactory stimuli [20].
2.9. Statistical Analysis
Results are expressed as Mean ± SEM. Comparison of the drug treated groups against vehicle treated group was made by one-way analysis of variance (ANOVA) followed by Dunnett’s t-test.
3. Results
3.1. Acute Toxicity
CFE was found to be non-toxic up to the dose 2000 mg/kg.
3.2. Effects of Acute CFE
3.2.1. Discrimination Index
Pre-treatment of mice with CFE 250, 500, 1000 mg/kg increased discrimination index in a dose-dependent manner compared to controls. Efficacy at the higher doses matched that of a reference dose of the recognised nootrope piracetam (Table 1).
3.2.2. Locomotor Activity
CFE at doses of 100 and 250 mg/kg did not significantly impact on locomotor activity. Higher doses (500 and 1000 mg/kg) reduced locomotor activity significantly (P < 0.05) (Figure 1).
3.3. Effects of Chronic CFE
3.3.1. Morris Water Maze Task Performance
In mice pre-treated with CFE at all doses, escape latency in both spatial reference and spatial working memory

Figure 1. Effect of CFE and diazepam on mean change in locomotor activity. n = 6 Data was analyzed by one-way ANOVA followed by Dunnett’s t test *P < 0.05, **P < 0.01.
Table 1. Effect of CFE and piracetam (PT) on discrimination index in object recognition test.
n = 6 Data was analyzed by one-way ANOVA followed by Dunnett’s t test. *P < 0.05, **P < 0.01.
models was significantly reduced (P < 0.01). Distance travelled by mice pre-treated with CFE 100 and 250 mg/kg in the spatial reference memory model and CFE 100 mg/kg in the spatial working memory model was also significantly reduced. This effect disappeared at higher doses, showing a biphasic effect common among anxiolytics (Figures 2 and 3).
3.3.2. Elevated Plus Maze Task Performance
Mice pre-treated with CFE 250, 500 and 1000 mg/kg significantly increased the time spent in open arm on the 2nd, 7th and 14th days, with a trend towards dose-dependency (Figure 4).
3.3.3. Radial Arm Maze Task Performance
CFE had no significant effects. The reference standard piracetam significantly reduced all learned parameters.
4. Discussion
Memory performance depends upon the type of difficulty
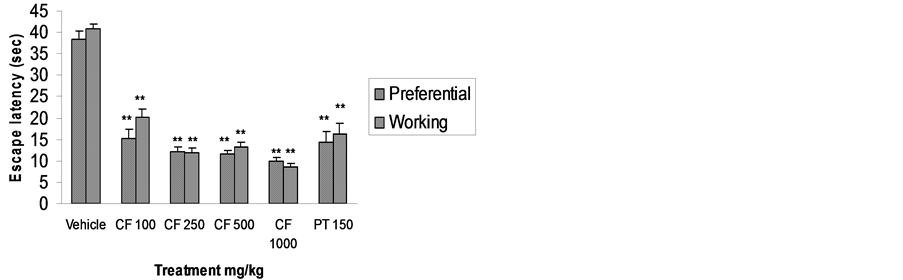
Figure 2. Effect of CFE and piracetam (PT) on escape latency using Morris water maze. n = 6 Data was analyzed by one-way ANOVA followed by Dunnett’s *P < 0.05, **P < 0.01.

Figure 3. Effect of CFE and piracetam (PT) on distance travelled using Morris water maze. n = 6 Data was analyzed by one-way ANOVA followed by Dunnett’s *P < 0.05, **P < 0.01.

Figure 4. Effect of CFE and piracetam (PT) on time spent in open arm using elevated plus maze. n = 6 Data was analyzed by one-way ANOVA followed by Dunnett’s *P < 0.05, **P < 0.01.
and the nature of task [18]; hence new drug evaluation is usually carried out using multiple models, as in our paper. The significant improvement in discrimination index in the object recognition test achieved by CFE indicated facilitation of learning and memory processes in the absence of cognitive deficit, a major criterion for classifying nootropic agents [21]. This supports its possible use in disorders related to episodic memory impairment associated with encoding and/or storage disorders and major perturbation of recollective judgment [22].
Mazes are traditionally used to evaluate spatial learning and memory. Spatial memory is a form of short term memory utilising neuro-circuitry that provides temporary storage and manipulation of information necessary for complex cognitive tasks such as language comprehension, learning and reasoning [23]. Its impairment is analogous to memory disorder in Alzheimer’s dementia [24].
CFE pre-treatment significantly reduced escape latency in Morris water maze performance, facilitating learning and memory processes integral to spatial navigation [20]. The reduction in distance travelled with lower doses of CFE does not meet the criteria of a classic nootropic agent as the effect disappeared at higher doses [20]. However, distance travelled can be reduced either via improved cognition or a change in swimming behaviour influenced by serotonergic transmission [25]. In the present investigation, a significant nootropic effect was observed with respect to escape latency with all doses of CFE. We believe that at higher doses, flavonoidal components in CFE may have exerted an inhibitory effect on swimming behaviour via serotonergic mechanisms [26], and will explore this in future work.
In the Elevated Plus Maze, mice pre-treated with CFE significantly increased time spent in the open arm, demonstrating an anxiolytic action [27] alongside CFE’s nootropic effects. This is the most important observation of the present investigation; anxiety is frequently associated with and can worsen impaired learning and weakened memory in Alzheimer’s disease, and there are currently no drugs that adequately treat co-presenting anxiety and cognitive deficit. Benzodiazepines, the most widely prescribed anxiolytic agents, have unfavourable effects on learning and memory processes [16,17] and selectively impair anterograde episodic memory [28]. Furthermore, benzodiazepines induce tolerance following repeated dosing [29] due to adaptive receptor changes in the central nervous system [30]. CFE pre-treatment showed significant and consistent anxiolytic action even after daily administration for 14 days, without behavioral toxicity; together with sustained cognitive enhancement.
Locomotor activity is considered to be an index of alertness and decreased activity indicates sedation. Locomotor activity was significantly decreased with CFE 500 and 1000 mg/kg pre-treatment, indicating a degree of sedation at the higher doses. Nootropic effects were shown at the lower two doses of CFE, which did not induce any sedation.
The radial arm maze (RAM) is used to evaluate hunger-motivated spatial memory in lab animals [20]. CFE did not impact on RAM performance at any dose, a null-result that had been anticipated due to CFE’s documented appetite-suppressant effects [31,32].
5. Conclusion
When administered chronically, CFE has significant nootropic and anxiolytic activity in mice. The combination of these usually mutually exclusive effects together with an absence of toxicity makes CFE an interesting candidate for the treatment of cognitive impairment copresenting with anxiety in the elderly; and its nootropic/anxiolytic/anorexogenic profile makes it a candidate treatment for Prader-Willi Syndrome also. Further work is underway to extend our understanding of its mechanism of action, including a study in aged animals.
Authors’ Contributions
NSV conceptualized and designed the study, drafted the manuscript, and has given final approval of the version to be published. RR, DBA, PRC and VDS made substantial contributions to the conception and design of the study and to the analysis and interpretation of data. RAK assisted with the acquisition of data and the statistical analysis. All authors read and approved the final manuscript.
Declaration of Interests
R. Rajendran is employed by Green Chem, which manufactured the CFE. P. Clayton provides occasional consultancy services to GenCor Pacific, which intends to develop CFE as a commercial product. Neither organization was involved in the preparation of this paper.
REFERENCES
- C. J. Vas, C. Pinto, D. Panniker, N. Deshpande, L. Kulkarni and S. Sachadeva, “Prevalence of Dementia in an Urban Indian Population,” International Psychogeriatrics, Vol. 13, No. 4, 2001, pp. 439-450. http://dx.doi.org/10.1017/S1041610201007852
- T. E. Andreoli, C. C. J. Carpenter, J. C. Bennett and F. Plum, “Cecil Essentials of Medicine,” 4th Edition, Saunders WB Co., Philadelphia, 2002, pp. 799-802.
- S. A. Beaudreau and R. O’Hara, “Late Life Anxiety and Cognitive Impairment: A Review,” American Journal of Geriatric Psychiatry, Vol. 16, No. 10, 2008, pp. 790-803. http://dx.doi.org/10.1097/JGP.0b013e31817945c3
- V. Ravichandra, A. Devi, S. Adiga and K. S. Rai, “Evaluation of the Effect of Glycerriza glabra Linn Root Extract on Spatial Learning and Passive Avoidance Response in Mice,” Indian Drug, Vol. 44, No. 3, 2007, pp. 214-219.
- A. D. Tarnalli and T. C. Cheeramkuzhy, “Influence of Clitoria Ternatea Extracts on Memory and Central Cholinergic Activity in Rats,” Pharmaceutical Biology, Vol. 38, No. 1, 2000, pp. 51-56.
- H. K. Singh and B. N. Dhavan, “Neuropharmacological Effect of Ayurvedic nootropic Baccopa monniera Linn,” Indian Journal of Pharmacology, Vol. 29, No. 5, 1997, pp. 259-265.
- J. N. Dhuley, “Nootropic Like Effect of Ashwagandha (Withania somniferra) in Mice,” Phytotherapy Research, Vol. 15, No. 6, 2001, pp. 524-528. http://dx.doi.org/10.1002/ptr.874
- K. Nalini, K. S. Karanth, A. Rao and A. R. Aroor, “Effects of Celastrus paniculatus on Passive Avoidance Performance and Biogenic Amine Turnover in Albino Rats,” Journal of Ethnopharmacology, Vol. 47, No. 2, 1995, pp. 101-108. http://dx.doi.org/10.1016/0378-8741(95)01264-E
- Y. Yamaguchi, M. Higashi and H. Kobayashi, “Effect of Ginsennosides on Impaired Performance Caused by Scopolamines in Rats,” European Journal of Pharmacology, Vol. 312, 1996, pp. 149-151. http://dx.doi.org/10.1016/0014-2999(96)00597-3
- A. Das, G. Shanker, N. Chandishwar, R. Pal, S. Singh and H. K. Singh, “A Comparative Study in Rodents of Standardized Extracts of Bacopa monniera and Ginko Biloba Anticholinesterase and Cognitive Enhancing Activities,” Pharmacology Biochemistry & Behavior, Vol. 73, No. 4, 2002, pp. 893-900. http://dx.doi.org/10.1016/S0091-3057(02)00940-1
- K. Kishore and N. Singh, “Effect of Bacosides, Alcoholic Extract of Bacopa monniera Linn (Brahmi), on Experimental Amnesis in Mice,” Indian Journal of Experimental Biology, Vol. 43, No. 7, 2005, pp. 640-645.
- A. Bader, A. Braca, N. De Tommasi and I. Morelli, “Further Constituents from Caralluma Negevensis,” Phytochemistry, Vol. 62, 2003, pp. 1277-1281. http://dx.doi.org/10.1016/S0031-9422(02)00678-7
- E. Aguirre-Hernández, M. E. González-Trujano, A. L. Martínez, J. Moreno, G. Kite, T. Terrazas and M. Soto-Hernández, “HPLC/MS Analysis and Anxiolytic-Like Effect of Quercetin and Kaempferol Flavonoids from Tilia Americana var. Mexicana,” Journal of Ethnopharmacology, Vol. 127, No. 1, 2010, pp. 91-97. http://dx.doi.org/10.1016/j.jep.2009.09.044
- Y. Hou, M. A. Aboukhatwa, D. L. Lei, K. Manaye, I. Khan and Y. Luo, “Anti-Depressant Natural Flavonols Modulate BDNF and Beta Amyloid in Neurons and Hippocampus of Double TgAD Mice,” Neuropharmacology, Vol. 58, No. 6, 2010, pp. 911-920. http://dx.doi.org/10.1016/j.neuropharm.2009.11.002
- L. Ren, F. Wang, Z. Xu, W. M. Chan, C. Zhao and H. Xue, “GABA(A) Receptor Subtype Selectivity Underlying Anxiolytic Effect of 6-Hydroxyflavone,” Biochemical Pharmacology, Vol. 79, No. 9, 2010, pp. 1337-1344. http://dx.doi.org/10.1016/j.bcp.2009.12.024
- M. Lader and S. Morton, “Benzodiazepine Problems,” British Journal of Addiction, Vol. 86, No. 7, 1991, pp. 823- 828. http://dx.doi.org/10.1111/j.1360-0443.1991.tb01831.x
- R. G. Lister, “The Use of Plus Maze to Measure Anxiety in Mouse,” Psychopharmacology, Vol. 92, No. 2, 1987, pp. 180-185. http://dx.doi.org/10.1007/BF00177912
- P. Quinette, B. Gaillery, B. Desgranges, V. Sayette, F. Vaider and F. Eustache, “Working Memory and Executive Function in Transient Global Amnesia,” Brain, Vol. 126, 2003, pp. 1917-1934. http://dx.doi.org/10.1093/brain/awg201
- G. Achliya, U. Barbade, S. Wadodkar and A. Dorle, “Effect of Brahmi Ghrita, a Polyherbal Formulation on Learning and Memory Paradigms in Experimental Animals,” Indian Journal of Pharmacology, Vol. 36, No. 3, 2004, pp. 159-162.
- H. G. Vogel and W. H. Vogel, “Drug Discovery and Evaluation: Pharmacological Assays,” 2nd Edition, Springer Ver Lag Berlin Heidelberg, New York, 2002, pp. 435- 436. http://dx.doi.org/10.1007/3-540-29837-1
- S. D. Iversen and S. H. Synder, Eds., “Handbook of Psychopharmacology,” Vol. 20, Plenum Press, New York, 1988, pp. 437-445.
- M. W. Brown and J. P. Aggelton, “Episodic Memory, Amnesia and the Hippocampalanterior Thalamic Axis,” Journal of Behavioral and Brain Science, Vol. 22, No. 4, 1998, pp. 425-444.
- M. Parle, D. Dhingra and S. K. Kulkarni, “Neurochemical Basis of Learning and Memory,” Indian Journal of Pharmaceutical Sciences, Vol. 66, No. 4, 2004, pp. 371-376.
- A. D. Baddeley, “Working Memory,” Science, Vol. 255, No. 5044, 1992, pp. 556-559. http://dx.doi.org/10.1126/science.1736359
- J. F. Cryan and I. Lucki, “Antidepressant Like Behavioral Effects Mediated by 5-Hydroxytryptamine2c Receptors,” Journal of Pharmacology and Experimental Therapeutics, Vol. 295, No. 3, 2000, pp. 1120-1126.
- Y. Pan, F. M. Wang, L. Q. Qiang, D. M. Zhang and L. D. Kong, “Icariin Attenuates Chronic Mild Stress-Induced Dysregulation of the LHPA Stress Circuit in Rats,” Psychoneuroendocrinology, Vol. 35, No. 2, 2010, pp. 272- 283. http://dx.doi.org/10.1016/j.psyneuen.2009.06.020
- S. Pellow, P. Chopin, S. E. File and M. Briley, “Validation of Open: Closed Arm Entries in an Elevated Plus Maze as a Measure of Anxiety in Rats,” Journal of Neuroscience Methods, Vol. 14, 1985, pp. 149-167. http://dx.doi.org/10.1016/0165-0270(85)90031-7
- R. Kumar, D. S. Mac and W. F. Gabrielli, “Anxiolytics and Memory: A Comparison of Lorazepam and Alprazolam,” Journal of Clinical Psychiatry, Vol. 48, No. 4, 1987, pp. 158-160.
- R. L. Smith and R. J. Barrett, “Tolerance to the Anticonflict Effects of Diazepam: Importance of Methodological Considerations,” Pharmacology Biochemistry & Behavior, Vol. 58, 1997, pp. 61-66. http://dx.doi.org/10.1016/S0091-3057(96)00460-1
- P. P. Roy-Byrne, “The GABA-Benzodiazepine Receptor Complex: Structure, Function, and Role in Anxiety,” Journal of Clinical Psychiatry, Vol. 66, Suppl. 2, 2005, pp. 14-20.
- R. Kuriyan, T. Raj, S. K. Srinivas, V. Mario, R. Rajendran and A. V. Kurpad, “Effect of Caralluma fimbriata Extract on Appetite, Food Intake and Anthropometry in Adult Indian Men and Women,” Appetite, Vol. 48, No. 3, 2007, pp. 338-344. http://dx.doi.org/10.1016/j.appet.2006.09.013
- S. Kamalakannan, R. Rajendran, R. Venkatesh, P. Clayton and A. K. Akbarsha, “Anti-Obesegenic and Anti-Atherosclerotic Properties of Caralluma fimbriata Extract,” Journal of Nutrition and Metabolism, 2011, Article ID: 285301.
NOTES
*Corresponding author.


